Figure 12. The secretory vesicle proteome for biosynthesis and secretion of active peptides.
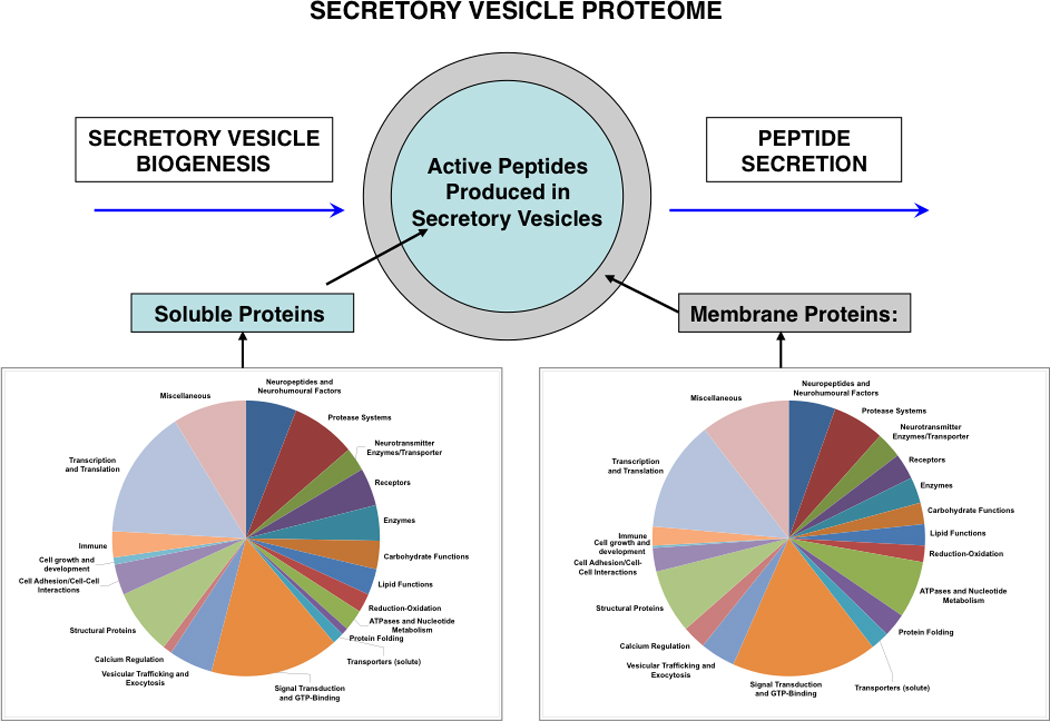
Proteins of the secretory vesicle, known as the neuroproteome, participate in the biosynthesis, storage, and regulated secretion of active peptides, as described in this review article. Thus, the active peptides are generated and secreted by the proteome of regulated secretory vesicles. The secretory vesicle proteome consists of soluble and membrane proteins that participate in secretory vesicle functions for providing neuropeptides for cell-cell communication in the nervous and endocrine systems. Proteomic studies of the soluble and membrane fractions of neuropeptide secretory vesicles isolated from adrenal medullary chromaffin cells of the sympathetic nervous system (bovine) indicate the protein systems participating in production of neuropeptides and β-amyloid for regulated secretion that include neuropeptides and neurohumoural factors, proteases, neurotransmitters enzymes and transporters, receptors, enzymes, carbohydrate functions, lipids, reduction-oxidation, ATPases and nucleotide metabolism, protein folding, signal transduction and GTP-binding proteins, vesicular trafficking and exocytosis, structural proteins, and cell adhesion proteins [111, 112].
