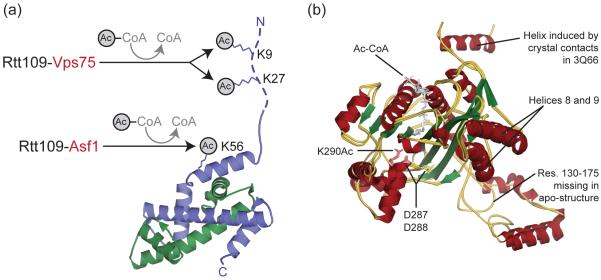
Figure 1. Rtt109 acetylates histone H3 at K9, K27 and K56.
(a) Rtt109-Vps75 acetylates K9 and K27 in the H3 tail (dotted line), while Rtt109-Asf1 acetylates K56 (shown as sticks) in the H3 histone-fold domain. Yeast H3 (blue) and H4 (green) are shown in cartoon (taken from nucleosome PDB 1ID3). (b) Rtt109 adopts a similar conformation when free (apo) or bound by Vps75. Shown is a cartoon superposition of free (PDB 3CZ7) and Vps75-bound Rtt109 (PDB 3Q66 and 3Q33) colored according to secondary structure. The acetyl-CoA and residues K290Ac, D287 and D288 are shown as sticks. Abbreviations: Res. – residues.