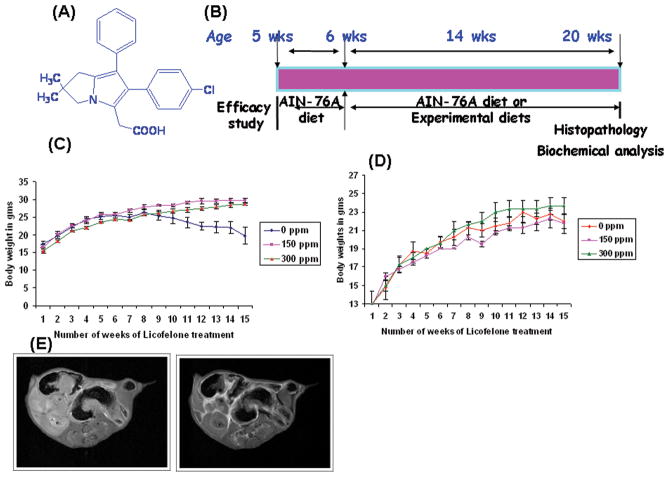
Figure 1.
A. Chemical structure of licofelone,(2-[6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1H-pyrrolizin-5-yl]acetic acid)
B. Experimental design for the evaluation of the chemopreventive efficacy of licofelone in APCmin/+ mice. Groups (10 mince/group) of mice were fed diets containing 0, 150 or 300 ppm licofelone from 6 weeks of age to the end of the experiment. The study was terminated after 100 days of exposure to the experimental diets. (See Materials and Methods for more details).
C & D. Changes in body weights over time for male (C) or female (D) mice fed control diets and/or experimental diets containing 150 or 300 ppm licofelone. Statistically significant differences in body weight gain were observed between licofelone-treated and control groups. Data are presented as Means ± SEM. Statistical Analysis was carried with ANOVA. Licofelone-treated animals were found to gain weight by the end of the study.
E. MRI of the small intestine in APCMin/+ mice. Small intestines of the treated and control animals were scanned for polyps by MRI. Comparing images from pre-and post-magnivest injection, polyps were identified as bright from accumulating magnivest. Left and right panels show polyp images in control and treated mice respectively.