Fig. 1.
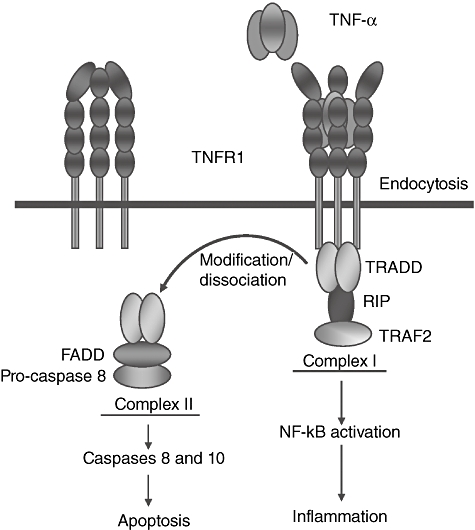
Tumour necrosis factor receptor 1 (TNFR1) and its signalling pathways. TNFR1 has four extracellular cysteine-rich domains (CRDs). Signalling via TNFR1 occurs by TNF-induced recruitment of monomeric membrane-bound TNFR1 molecules to form a homotrimer configuration which, when bound to ligand, causes a three-dimensional conformational change in the extracellular domain that is then transduced intracellularly to activate signalling pathways. The cytoplasmic tail of TNFR1 contains a TNF receptor type 1-associated death domain (TRADD) that couples this receptor to one of two distinct signalling pathways. The default pathway activates nuclear factor kappa-B (NF-κB) and other non-apoptotic pathways (not shown). This is mediated by the formation of complex I, comprising TNFR1 and NF-kB activating signalling components such as receptor interacting protein (RIP) and TNF receptor-associated factor 2 (TRAF2). Formation of complex I is transient and TNFR1 can be internalized after TNF binding leading to dissociation of the TRADD/TRAF2/RIP complex and association of FADD, recruitment of pro-caspase 8 and formation of complex II, which then recruits the apoptotic machinery. If NF-kB activation triggered by complex I is successful, cellular FLIPLlevels (see below) are sufficiently elevated to block apoptosis and cells survive. The ovals in the figure represent different activating signalling components. FADD: FAS-associated death domain. FLIPL: FLICE-like inhibitory protein long, the long form of caspase 8 inhibitor.
