Abstract
Objective
It is generally accepted that patients who require biventricular mechanical support (BiVAD) have poorer outcomes than those requiring isolated left ventricular support (LVAD). However, it is unknown how the timing of BiVAD insertion affects outcomes. We hypothesized that planned BiVAD insertion improves survival compared to delayed conversion of LVAD to BiVAD.
Methods
We reviewed and compared outcomes of 266 patients undergoing LVAD or BiVAD placement at the University of Pennsylvania from April 1995 to June 2007. We subdivided BiVAD patients into planned BiVAD (P-BiVAD) and delayed BiVAD (D-BiVAD) groups, based on the timing of RVAD insertion. We defined D-BiVAD as any failure of isolated LVAD support.
Results
Of 266 LVAD patients, 99 required BiVAD (37%). We compared preoperative characteristics, successful bridging to transplant, survival to hospital discharge, and Kaplan-Meier one-year survival between P-BiVAD (n=71) and D-BiVAD (n=28) groups. Preoperative comparison showed that patients who ultimately require biventricular support have similar preoperative status. LVAD (n=167) outcomes in all categories exceeded both P-BiVAD and D-BiVAD outcomes. Further, P-BiVAD patients had superior survival to discharge than D-BiVAD patients (51% v 29% p<0.05). One-year and long-term Kaplan-Meier survival distribution confirmed this finding. There was also a trend towards improved bridging to transplant in P-BiVAD (n=55) vs. D-BiVAD (n=22) patients (65% v 45% p=0.10).
Conclusion
When patients at risk for isolated LVAD support failure are identified, proceeding directly to BiVAD implantation is advised, as early institution of biventricular support results in dramatic improvement in survival.
Background
Early morbidity and mortality in mechanical circulatory support device recipients results primarily from multiple organ failure, postoperative hemorrhage, pulmonary complications, and thromboembolic events.1–9 Rates of these complications are significantly higher in patients who require biventricular support. In fact, right ventricular (RV) failure requiring ventricular assist device (RVAD) placement is the most significant risk factor for mortality in left ventricular assist device (LVAD) recipients.9
Numerous investigators have reported poor outcomes in LVAD patients with significant RV dysfunction. Santambrogio reported an 85% transplantation rate among LVAD recipients without RV failure, and only a 25% transplantation rate in LVAD recipients with RV failure.10 Dang showed similarly poor survival to transplantation among patients receiving RVADs after LVADs (35.7%), compared to LVAD recipients without RV failure (89.9%).11 Farrar reported a 58% transplantation rate with Thoratec BiVADs, as compared with a 74% transplantation rate among Thoratec LVAD recipients.12 Finally, Kormos and colleagues reported a 100% transplantation rate in LVAD recipients without RV failure, while their BiVAD recipients survived to transplant in only 40% of cases.13 These studies had relatively small sample sizes, with the exception of the multi-center Thoratec study,12 which was obviously subject to significant variability in practice and outcomes between sites.
Clearly, the elevated morbidity and mortality in post-LVAD RV failure mandates preoperative identification of patients who require biventricular support. A small number of studies have attempted to characterize preoperative parameters which can be utilized to predict which patients require biventricular mechanical support (BiVAD).10–16 In total the published literature identifies at least 25 different potential predictors of severe RV failure in LVAD recipients, including but not limited to: low right ventricular stroke work index (RVSWI), preoperative mechanical ventilation, elevated creatinine, female gender, and small body surface area. To enhance the understanding of this problem, we studied our LVAD and BiVAD cohort at the Hospital of the University of Pennsylvania to identify preoperative predictors of BiVAD need. We found and reported that low cardiac index, low RVSWI, echocardiographic evidence of severe pre-VAD RV dysfunction, elevated creatinine, previous cardiac surgery, and low systolic blood pressure are independently associated with BiVAD use.17 Though a widely accepted consensus does not yet exist, these studies establish that it is possible to preoperatively identify patients who require biventricular mechanical circulatory support.
If it is possible to predict which patients require BiVADs, then it is plausible that early, planned institution of biventricular support will result in better outcomes than delaying insertion of the RVAD. Two studies have reported results with patients who received planned BiVADs. Magliato showed 59% survival to transplantation among 17 Thoratec BiVAD recipients,18 while Tsukui reported an exceptional 84% transplantation rate in select patients who received planned BiVADs.19 These results represent substantial improvements over reported outcomes in patients who had delayed conversion of LVAD to BiVAD.10–13
The reports by Magliato and Tsukui lead us to believe that timely institution of biventricular support is the most effective strategy for patients at risk for LVAD support failure. Unfortunately, neither of these reports compares planned BiVAD outcomes with unplanned BiVAD outcomes at their institutions. Accordingly, we studied our cohort of 266 LVAD recipients, 99 of whom also required mechanical support of the RV, in order to compare outcomes between LVAD, planned BiVAD, and delayed BiVAD recipients. This represents the largest single-institution series to address this question.
Hypothesis
We hypothesized that patients at high risk for LVAD support failure can achieve improved outcomes with early, planned BiVAD insertion as opposed to delayed conversion of LVAD to BiVAD.
Methods
Patients
We performed a retrospective review on all patients who underwent LVAD implantation at the Hospital of the University of Pennsylvania from April 1995 to June 2007. Patients were assigned to separate LVAD and BiVAD groups for comparison. Based on the timing of device insertion, the BiVAD group was further divided into two subgroups: 1.) Pre-planned BiVAD support (P-BiVAD group); or 2.) Delayed conversion of LVAD to BiVAD (D-BiVAD group).
The P-BiVAD group included only patients who were taken to the operating room with a predetermined plan to place biventricular support devices. The devices were implanted concurrently and no attempt to utilize isolated LVAD support was made. D-BiVAD was defined as any failure of isolated LVAD support requiring conversion to BiVAD. This included patients who had LVADs converted to BiVADs: 1.) During two separate operations; or 2.) During the same operation because they could not be weaned from cardiopulmonary bypass (CPB) and taken to the intensive care unit on LVAD support alone. Though these scenarios differ somewhat, they both represent unplanned conversion of an LVAD to a BiVAD.
The decision to implant an RVAD was made by the individual cardiac surgeon in consultation with the heart failure cardiologist. Multiple factors influenced this decision. In addition to clinical status, preoperative considerations include patient size, transplant eligibility, device availability, and expected duration of support. At our institution, transplant ineligible patients are considered inappropriate for long-term RVAD use. However, if a brief period of RV support is anticipated, a temporary RVAD can be used. Furthermore, the choice of LV device influences the use of an RV device, by potentially altering the need for anticoagulation or the patient’s eligibility for outpatient therapy.
Thirty-seven percent of our cohort required biventricular support. Two published series report similar or greater proportions of patients who required BiVADs,12, 13 though most series have a smaller percentage. In our institution, all LVAD recipients at risk for RV failure are started on a milrinone infusion and inhaled prostacyclin to optimize the function of the RV prior to weaning from cardiopulmonary bypass. If, upon weaning from bypass, the hemodynamic situation is still not satisfactory, we proceed to RVAD implantation. The higher proportion of RVAD use in our cohort is explained by multiple factors. First, most series report only patients who failed LVAD support and then required RVAD support, thereby excluding patients who received pre-planned BiVADs. Additionally, our BiVAD cohort may represent a population more likely to require RV support than those reported in other studies, evidenced by the high proportion of females (37%), preoperative mechanical ventilation (71%), intra-aortic balloon pump (61%), and ECMO (21%), in our cohort.
Devices
Multiple devices were used throughout the study period. These include the BioMedicus Perfusion System (Medtronic, Inc., Littleton, MA), TCI IP (Thermo Cardiosystems, Inc., Woburn, MA), TCI VE (Thermo Cardiosystems), HeartMate XVE (Thoratec Corp., Pleasonton, CA), Abiomed BVS-5000 (Abiomed, Inc., Danvers, MA), Thoratec PVAD (Thoratec), and HeartMate II (Thoratec). We define the BioMedicus and Abiomed as short-term devices, while the Thoratec PVAD, TCI IP, TCI VE, and HeartMate VADs are defined as long-term devices. In the BiVAD subcohorts, the use of short- and long-term devices was statistically equivalent, eliminating an inferior device as an explanation for poor outcomes among the D-BiVAD group.
Data
The study protocol was approved by the institutional review board (IRB) of the University of Pennsylvania. Data was collected retrospectively from the medical records. Preoperative data including demographics, clinical factors, hemodynamic parameters, and laboratory values were entered into a database. These data were used to establish the baseline characteristics of the two BiVAD groups. Date of device implantation, date of transplant, date of initial hospital discharge, current status (alive or deceased), and date of death were also collected. These data points allowed us to compare survival to hospital discharge, survival to transplant, and one-year and long-term Kaplan-Meier survival distribution between the LVAD, P-BiVAD, and D-BiVAD groups. Following accrual of data, identifying information was removed from the database and a unique code number was assigned to each record.
Statistical Analysis
SAS 9.1.3 (SAS Institute, Cary, NC) was used to perform statistical analysis. Preoperative parameters were compared using X2 tests for categorical variables, and unpaired student t-tests for continuous variables. Patients requiring preoperative mechanical circulatory support were excluded from analysis of hemodynamic variables.
Survival to hospital discharge and survival to transplantation were compared between LVAD, P-BiVAD, and D-BiVAD groups using Χ2 tests. Kaplan-Meier survival distribution was compared between the three groups using XLSTAT version 2007.6 (Addinsoft USA, New York, NY) statistical analysis add-in software for Microsoft Excel. For all statistical analyses, the level of significance was P ≤ 0.05.
Results
There were 266 patients who received LVADs at the Hospital of the University of Pennsylvania during the twelve year study period. Of these, 167 patients (63%) tolerated isolated LVAD support, while 99 patients (37%) required biventricular support. Of the 99 patients who received BiVADs, 71 patients underwent pre-planned BiVAD insertion (P-BiVAD), while 28 patients underwent delayed conversion of LVAD to BiVAD (D-BiVAD).
Of the 28 D-BiVADs, 14 patients underwent RVAD insertion during the LVAD operation, due to severe RV failure in the setting of hemodynamic instability with isolated LVAD support. The remaining 14 D-BiVAD patients underwent RVAD implantation during a later operation, due to progressive multi-organ dysfunction resulting from severe RV failure. The median time to RVAD implantation in this subgroup was 2 days. Outcomes in all parameters were equivalent in these two D-BiVAD subgroups.
Results of univariate analysis comparing the P-BiVAD and D-BiVAD subgroups are shown in Table 1. Of the 35 parameters compared between the two groups, only heart rate (HR) and diastolic blood pressure (DBP) were significantly different between the two groups. Unexpectedly, HR was slightly higher and DBP slightly lower in the group that received unplanned BiVADs. This is counterintuitive if one presumes that patients exhibiting higher HR and lower DBP would be considered more ill, and would therefore receive BiVADs in a planned fashion. Multivariate comparison of P-BiVAD and D-BiVAD groups was not performed due to the lack of significant preoperative differences between the populations.
Table 1.
Univariate Comparison of Preoperative Characteristics between Planned BiVAD and Delayed BiVAD Recipients.
| Variable | P-BiVAD (n=71) | D-BiVAD (n=28) | P-value |
|---|---|---|---|
| Age (years) | 52.4 ± 10.9 | 49.3 ± 13.2 | 0.2787 |
| Gender (% female) | 37 | 39 | 0.8050 |
| Body Surface Area (m2) | 1.92 ± 0.25 | 1.95 ± 0.29 | 0.7104 |
| Ischemic Cardiomyopathy (vs. Non-Ischemic, %) | 59 | 53 | 0.6127 |
| COPD (%) | 8 | 7 | 0.8297 |
| Diabetes (%) | 25 | 29 | 0.7430 |
| Mechanical Ventilation (%) | 70 | 71 | 0.9211 |
| Acute Myocardial Infarction (%) | 27 | 21 | 0.5823 |
| Previous Cardiac Surgery (%) | 38 | 54 | 0.1587 |
| Severe Preoperative RV Dysfunction (%)1 | 75 | 68 | 0.4753 |
| Intra-aortic Balloon Pump (%) | 56 | 71 | 0.1664 |
| Preoperative Circulatory Support (%) | 20 | 25 | 0.5626 |
| Non-separation from Cardiopulmonary Bypass (%) | 23 | 21 | 0.9051 |
| Heart Rate (beats/minute) | 100.1 ± 21.6 | 113.9 ± 22.4 | 0.0299 |
| Systolic Blood Pressure (mmHg) | 96.9 ± 17.5 | 92.2 ± 11.5 | 0.2010 |
| Diastolic Blood Pressure (mmHg) | 57.5 ± 12.5 | 51.2 ± 10.2 | 0.0389 |
| Mean Arterial Blood Pressure (mmHg) | 70.6 ± 13.0 | 64.9 ± 9.9 | 0.0565 |
| Central Venous Pressure (mmHg) | 22.5 ± 8.2 | 21.6 ± 6.5 | 0.6824 |
| Pulmonary Artery Systolic Pressure (mmHg) | 46.0 ± 11.8 | 41.1 ± 10.2 | 0.1151 |
| Pulmonary Artery Diastolic Pressure (mmHg) | 26.5 ± 8.7 | 26.2 ± 7.4 | 0.8793 |
| Mean Pulmonary Artery Pressure (mmHg) | 33.0 ± 9.0 | 31.2 ± 7.9 | 0.4278 |
| Cardiac Index (L/min/m2) | 1.77 ± 0.44 | 1.85 ± 0.36 | 0.4774 |
| SvO2 (%) | 56.8 ± 13.0 | 54.5 ± 9.3 | 0.4842 |
| Right Ventricular Stroke Work Index (mmHg·L/m2) | 0.195 ± 0.201 | 0.209 ± 0.178 | 0.8046 |
| LV Ejection Fraction (%) | 15.0 ± 11.9 | 14.0 ± 17.0 | 0.7601 |
| White Blood Cell Count (109/L) | 14.0 ± 7.4 | 11.5 ± 5.1 | 0.0788 |
| Hemoglobin (g/dL) | 11.3 ± 1.9 | 11.2 ± 2.0 | 0.7989 |
| Platelet Count (109/L) | 183.9 ± 109.9 | 165.0 ± 79.5 | 0.3692 |
| International Normalized Ratio | 1.76 ± 0.86 | 1.87 ± 1.11 | 0.6510 |
| Partial Thromboplastin Time (seconds) | 56.3 ± 33.7 | 53.8 ± 32.8 | 0.7500 |
| Creatinine (mg/dL) | 2.08 ± 1.39 | 1.92 ± 1.46 | 0.6271 |
| Total Bilirubin (mg/dL) | 1.56 ± 1.48 | 1.85 ± 1.68 | 0.4898 |
| Alanine Aminotransferase (U/L) | 626.4 ± 1632.8 | 253.5 ± 687.8 | 0.1993 |
| Aspartate Aminotransferase (U/L | 632.2 ± 1732.7 | 268.7 ± 309.9 | 0.1460 |
| Albumin (g/dL) | 2.7 ± 0.6 | 2.7 ± 0.8 | 0.8395 |
RV dysfunction was graded as none, mild, moderate, or severe, and was based on the final report of the preoperative echocardiogram.
Right Ventricular Stroke Work Index = (mean PAP − CVP) × Cardiac Index/Heart Rate.
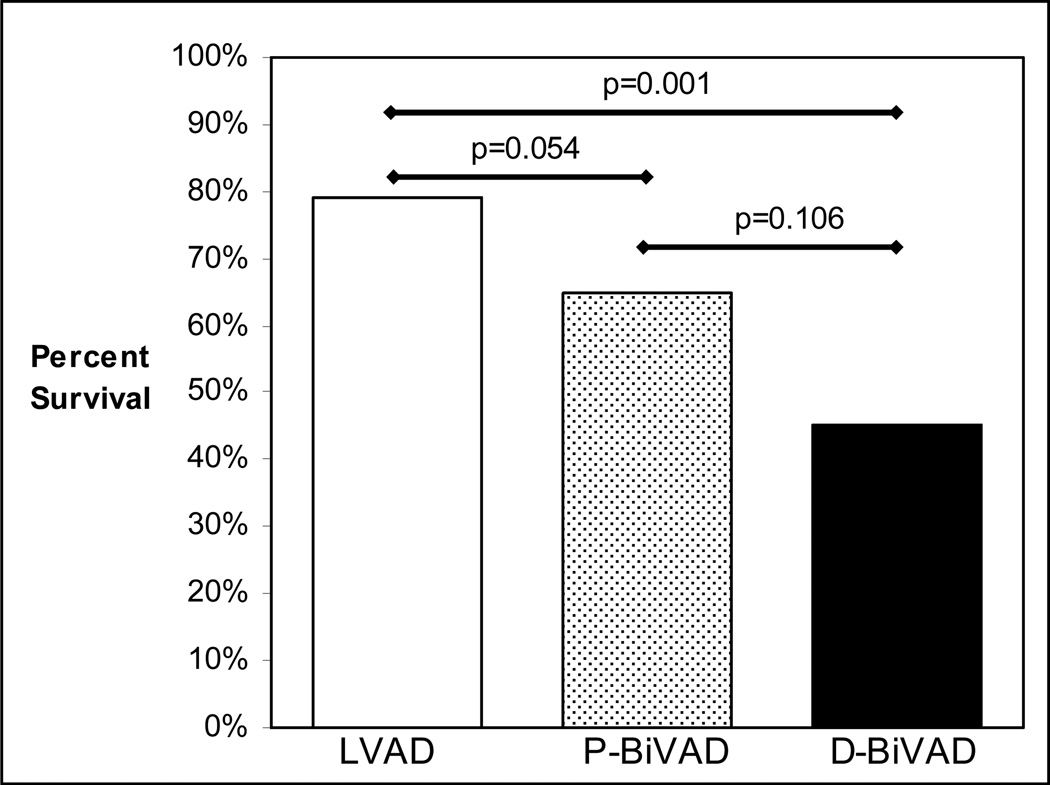
Transplant eligible patients in each of the three study groups were identified by excluding patients who received LVADs as destination therapy, or who were successfully recovered from VAD support. All other patients were considered transplant eligible regardless of whether they were ever listed for transplantation. Of 141 transplant eligible patients in the LVAD group, 111 were ultimately transplanted (79%). This proportion was not significantly higher than the transplantation rate in the P-BiVAD group (65%, 36 of 55, P=0.0539). Expectedly, the 79% transplantation rate in the LVAD group was significantly greater than the 45% survival to transplant achieved in the D-BiVAD group (10 of 22, P=0.0009). Importantly, patients who underwent planned BiVAD insertion trended towards better survival to transplantation than patients undergoing delayed BiVAD insertion (65% vs. 45%, P=0.1). Survival to transplantation among the three groups is shown in Figure 1.
Figure 1.
Survival to Cardiac Transplantation compared between LVAD, P-BiVAD, and D-BiVAD groups.
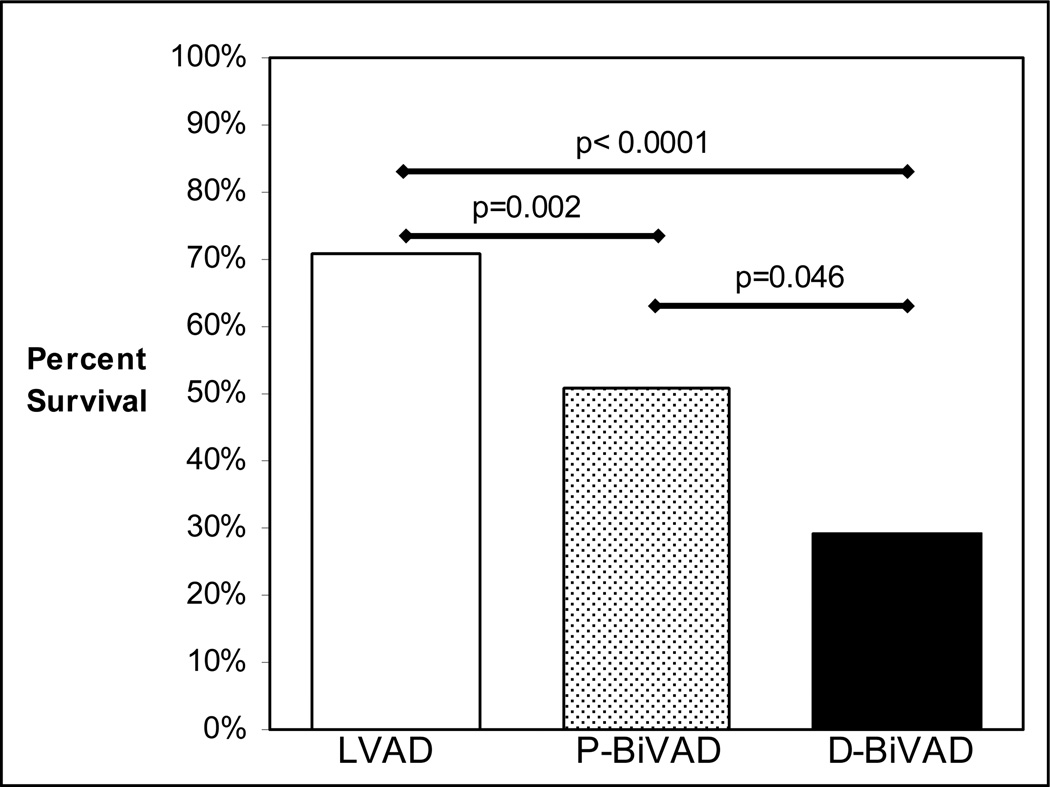
Survival to hospital discharge (Figure 2) was highest among the LVAD group (71%, 119 of 167). This was significantly higher than survival to hospital discharge in both the P-BiVAD group (51%, 36 of 71, P=0.0023), and the D-BiVAD group (29%, 8 of 28, P<0.0001). Most importantly, when P-BiVADs (51% survival) were compared to D-BiVADs (29% survival), the result remained statistically significant (P=0.0459). In a further subgroup analysis, survival to hospital discharge was determined for the most commonly employed devices. When the HeartMate I (TCI IP, TCI VE, or HeartMate XVE) was chosen as the LVAD, survival to discharge was 74.4% (87 of 117 patients) for isolated LVAD recipients, 71.4% (5 of 7) for P-BiVAD recipients, and 28.6% (4 of 14) for D-BiVAD recipients. When a Thoratec was chosen as the LVAD, survival to discharge was 63.2% (24 of 38) for isolated LVAD recipients, 56.0% (28 of 50) for P-BiVAD recipients, and 36.4% (4 of 11) for D-BiVAD recipients. These results further support the assertion that survival is primarily determined by the initial decision to implant uni- or biventricular devices, rather than the choice of device type.
Figure 2.
Survival to Hospital Discharge compared between LVAD, P-BiVAD, and D-BiVAD groups.
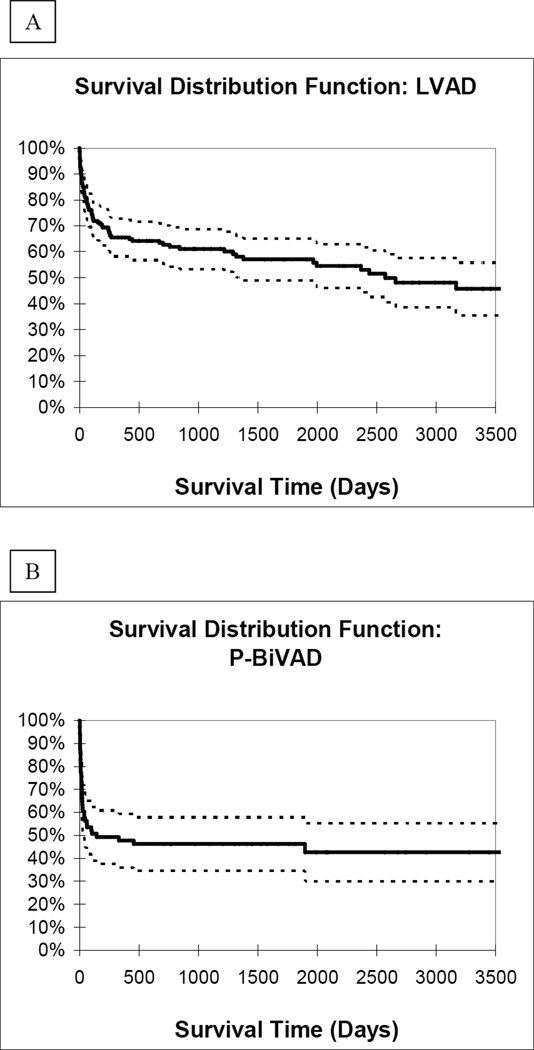
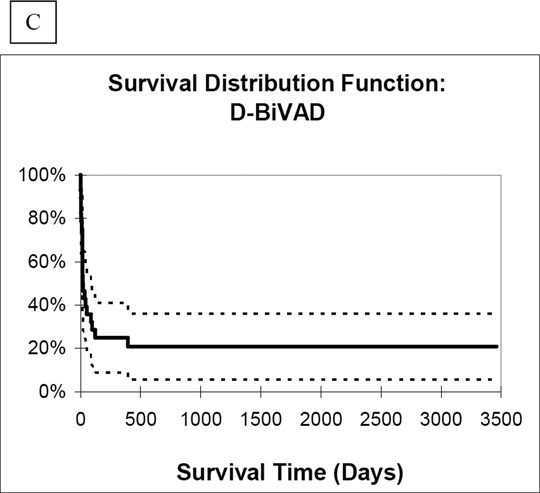
Analysis of Kaplan-Meier one year survival distribution likewise showed the best results in the LVAD group (65%). When compared to the P-BiVAD group, which had 48% one-year survival, this result was statistically significant (P=0.003). One-year survival was lowest in the D-BiVAD group, at 25%, also significantly lower than the LVAD group (P<0.0001). Importantly, comparison between the two BiVAD groups showed statistically higher one-year survival among P-BiVAD recipients (P=0.025). Finally, long-term survival distribution curves (Figure 3) showed similar results among the three study groups (LVAD vs. P-BiVAD, P=0.014; LVAD vs. D-BiVAD, P<0.0001; P-BiVAD vs. D-BiVAD, P=0.019).
Figure 3.
Long-term survival distribution curves for A.) LVAD, B.) P-BiVAD, and C.) D-BiVAD groups.
Discussion
Patients who require mechanical circulatory support face the risk of life-threatening postoperative complications, which they must overcome in order to achieve long-term survival. The development of severe RV failure after LVAD implantation is a serious complication, resulting in end-organ dysfunction in multiple organ systems. Multi-organ failure subsequently results in dramatically increased mortality in this group.
The most effective therapy for these patients is biventricular mechanical support. However, it is now well-recognized that BiVAD recipients have higher morbidity and mortality rates than LVAD recipients in nearly all categories. Because destination therapy is generally not an option for BiVAD patients, long-term survival among BiVAD recipients is closely tied to their ability to survive to transplantation, or recover from VAD support.
Fortunately, post-transplant survival does not appear to be linked to the use of univentricular or biventricular preoperative support. Farrar showed equivalent post-transplantation survival through hospital discharge between Thoratec LVAD and BiVAD groups,12 and the Columbia University group has shown that pre-transplant RVAD support was not a risk factor for post-transplant mortality in their cohort.11,14 Additionally, Magliato reported 90% post-transplant survival to hospital discharge in planned BiVAD recipients.18
Therefore, if cardiac transplantation is the event that equalizes survival between LVAD and BiVAD patients, we must improve the rate at which BiVAD recipients survive to transplant. Because their morbid illness results from severe biventricular failure, it is logical to assume that timely restoration of cardiac output will improve the ability of BiVAD recipients to survive the perioperative period. Magliato and Tsukui have reported favorable results in patients who underwent planned BiVAD insertion, however the current literature does not adequately address how the timing of BiVAD placement affects patient outcomes.
The current study is the largest to directly compare results at a single institution between planned BiVAD outcomes and delayed BiVAD outcomes. In addition, no other study has examined whether unplanned RVAD insertion immediately after LVAD insertion (during the same operation) has a deleterious effect on patient outcomes.
Our previous study established that preoperative demographics, clinical factors, hemodynamic data, and laboratory values can be used to readily distinguish between patients who tolerate isolated LVAD support and those who ultimately require biventricular support.17 In the current study, we compared preoperative parameters between P-BiVAD recipients and D-BiVAD recipients to determine if the D-BiVAD group exhibited characteristics suggesting they would tolerate LVAD support. Surprisingly, the two groups were nearly equivalent, as only heart rate and diastolic blood pressure showed statistically significant differences. Additionally, the small heart rate elevation and diastolic blood pressure decrease observed in D-BiVAD recipients are probably not meaningful clinically. In summary, patients who require biventricular support can be clearly distinguished based on preoperative characteristics from those who tolerate isolated LVAD support; further, there is reason to believe that all patients who ultimately receive BiVADs are equally identifiable prior to VAD insertion, based on critical analysis of preoperative data.17
With respect to outcomes, LVAD recipients survived to transplant at the highest rate (79%). However, planned BiVAD recipients in our cohort survived to transplant in 65% of cases, a result which was not significantly lower than the transplant rate in LVAD recipients. This bridge to transplantation rate among P-BiVAD patients is at least comparable to, if not better than, rates published by Morgan, Magliato, and Tsukui.14,18,19 Similarly, our 45% bridge to transplantation rate among D-BiVAD patients compares with the poor results in other published series. The fact that survival to transplantation among P-BiVADs was not significantly higher than that among D-BiVADs is likely due to the small number of transplant eligible patients who received D-BiVADs (n=22). Nonetheless, these results clearly argue in favor of a strategy of P-BiVAD placement for patients who require biventricular support.
Not unexpectedly, survival to hospital discharge was highest in the LVAD group (71%). Importantly, P-BiVAD recipients survived to hospital discharge at a significantly higher rate (51%) than those who had BiVADs placed in a delayed fashion (29%). This represents a relative in-hospital mortality reduction of 31% in the P-BiVAD group, compared to the D-BiVAD group. The fact that survival to hospital discharge was significantly higher among P-BiVADs than D-BiVADs, even though the transplantation rate was not significantly different, is explained by the fact that a high proportion of P-BiVAD recipients (16 of 71, 23%) were successfully recovered from VAD support, and therefore did not require transplantation.
Survival distribution rates demonstrate similar results. Kaplan-Meier one year survival again was highest in the LVAD group (65%). Notably, one-year survival was significantly higher in the P-BiVAD group (48%) than the D-BiVAD group (25%). Again, this represents a 31% relative risk reduction for one-year mortality in the P-BiVAD group, as compared to the D-BiVAD group. Most importantly, long-term Kaplan Meier survival distribution mirrored the findings at one year. Importantly, if one examines BiVAD recipients at our institution, those who were appropriately identified as requiring biventricular support prior to initial VAD placement survived to hospital discharge and to one year at nearly twice the rate as those who were not initially believed to require BiVADs. This study therefore provides strong evidence that a strategy of early, planned BiVAD insertion for patients who require biventricular mechanical circulatory support results in higher transplantation rates and improved long-term survival.
The factors which result in the increased mortality in the BiVAD groups should ideally be determined. Because VAD recipients develop complications in multiple organ systems and the definition of these may vary, it was difficult in this retrospective review to definitively identify every single complication. Nonetheless, reoperation rates (P-BiVAD 44 of 71, 62%; D-BiVAD 21 of 28, 75%) and the need for postoperative hemodialysis (P-BiVAD 22 of 71, 31%; D-BiVAD 11 of 28, 39%) were similar between the BiVAD subcohorts. The majority of reoperations were attributed to bleeding. In the BiVAD subcohorts, patients who died in the hospital had similar survival time (P-BiVAD: mean 20.2 days, median 15 days; D-BiVAD: mean 22.4 days, median 14.5 days). Though many factors were involved in the survival time of these patients, causes of death were likewise similar between the BiVAD subcohorts. Patients who died early after BiVAD generally died of progressive multi-organ dysfunction, while those who survived longer died as a result of infection, stroke, and debilitation. These facts demonstrate that P-BiVAD and D-BiVAD patients develop similar postoperative complications, but patients who receive planned BiVADs have a tendency to survive at a higher rate.
Limitations
This was a retrospective study performed at a single institution. The timing of device implantation and the device type were based on the clinical judgment of the surgeon in consultation with the heart failure cardiologists, as opposed to a defined protocol. Additionally, there are innumerable factors which influence the decision to utilize univentricular versus biventricular support. Finally, some percentage of RV failure is related to intraoperative events that may not be predictable preoperatively. These include lung injury, massive transfusion, and poor intraoperative RV protection. There was no way to correct for the presence of these factors in this study.
Conclusions
Exceedingly high postoperative morbidity and mortality present a significant challenge to long-term survival in patients who require biventricular mechanical circulatory support. These poor outcomes result primarily from end-organ dysfunction due to the severity of the biventricular failure. This study strongly supports a strategy of early, planned institution of biventricular mechanical circulatory support to improve survival in patients with morbid heart failure. In fact, with this strategy it may be possible for patients who receive planned BiVADs to achieve outcomes comparable to those of LVAD recipients. Further, this study highlights the importance of establishing widely accepted criteria to preoperatively identify patients who require biventricular support so they can receive appropriate support devices.
Acknowledgments
Funding: Y.J.W. is supported in part by NIH HL072812. J.R.F. III is supported by an ISHLT Research Fellowship Grant. J.R.F. is supported by NIH HL07843.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339(21):1522–1533. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 2.Rao V, Oz MC, Flannery MA, Catanese KA, Argenziano M, Naka Y. Revised screening scale to predict survival after insertion of a left ventricular assist device. J Thorac Cardiovasc Surg. 2003;125:855–861. doi: 10.1067/mtc.2003.111. [DOI] [PubMed] [Google Scholar]
- 3.Potapov EV, Loforte A, Weng Y, Jurmann M, Pasic M, Drews T, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg. 2008;23(3):185–194. doi: 10.1111/j.1540-8191.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson KD, Eppinger MJ, Dyke DB, Wright S, Pagani FD. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol. 2002;9:1247–1254. doi: 10.1016/s0735-1097(02)01751-5. [DOI] [PubMed] [Google Scholar]
- 5.Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, Tierney AR, et al. The REMATCH trial: rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann Thorac Surg. 1999;67(3):723–730. doi: 10.1016/s0003-4975(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 6.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 7.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, et al. Outcomes of left ventricular assist device implantation as destination therapy in the Post-REMATCH era: Implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA. Mechanical circulatory support: New data, old problems. Circulation. 2007;116:461–462. doi: 10.1161/CIRCULATIONAHA.107.715300. [DOI] [PubMed] [Google Scholar]
- 9.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos RL, et al. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: Third annual report-2005. J Heart Lung Transplant. 2005;24:1182–1187. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Santambrogio L, Bianchi T, Fuardo M, Gazzoli F, Veronesi R, Braschi A, et al. Right ventricular failure after left ventricular assist device insertion: preoperative risk factors. Interact CardioVasc Thorac Surg. 2006;5:379–382. doi: 10.1510/icvts.2006.128322. [DOI] [PubMed] [Google Scholar]
- 11.Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Farrar DJ, Hill JD, Pennington DG, McBride LR, Holman WL, Kormos RL, et al. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the Thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113:202–209. doi: 10.1016/S0022-5223(97)70416-1. [DOI] [PubMed] [Google Scholar]
- 13.Kormos RL, Gasior TA, Kawai A, Pham SM, Murali S, Hattler BG, et al. Transplant candidate’s clinical status rather than right ventricular function defines need for univentricular versus biventricular support. J Thorac Cardiovasc Surg. 1996;111:773–783. doi: 10.1016/s0022-5223(96)70337-9. [DOI] [PubMed] [Google Scholar]
- 14.Morgan JA, John R, Lee BJ, Oz MC, Naka Y. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg. 2004;77:859–863. doi: 10.1016/j.athoracsur.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation. 2002;106:I-198–I-202. [PubMed] [Google Scholar]
- 16.Fukamachi K, McCarthy PM, Smedira NG, Vargo RL, Starling RC, Young JB. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181–2184. doi: 10.1016/s0003-4975(99)00753-5. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick JR, Frederick JR, Hsu VM, Kozin ED, O'Hara ML, Howell E, et al. A risk score derived from preoperative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008 doi: 10.1016/j.healun.2008.09.006. (Full length manuscript accepted, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magliato KE, Kleisli T, Soukiasian HJ, Tabrizi R, Coleman B, Hickey A, et al. Biventricular support in patients with profound cardiogenic shock: A single center experience. ASAIO J. 2003;49(4):475–479. [PubMed] [Google Scholar]
- 19.Tsukui H, Teuteberg JJ, Murali S, McNamara DM, Buchanan JR, Winowich S, et al. Biventricular assist device utilization for patients with morbid congestive heart failure: A justifiable strategy. Circulation. 2005;112 suppl I:I-65–I-72. doi: 10.1161/CIRCULATIONAHA.104.524934. [DOI] [PubMed] [Google Scholar]