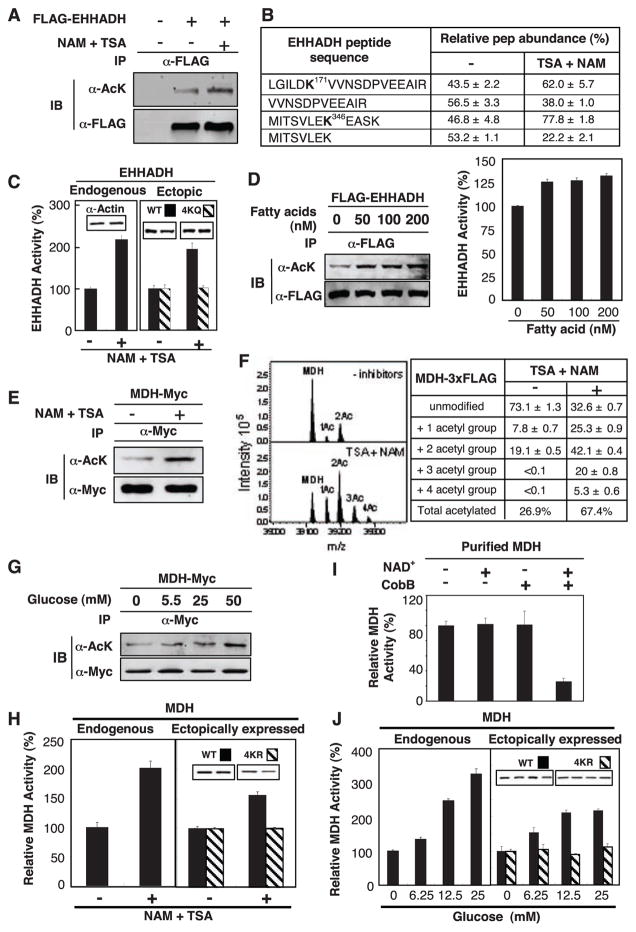
Fig. 2.
Activation of EHHADH and MDH by acetylation. (A) Acetylation of EHHADH was increased by deacetylase inhibitors. Ectopically expressed and immunoprecipitated (IP) EHHADH was examined by immunoblotting (IB) with antibody to acetyllysine (α-AcK). (B) Quantification of EHHADH acetylation by iTRAQ MS. Quantification of peptides was calculated on the basis of relative intensity of the iTRAQ tags. (C) Activation of EHHADH in cells expose to deacetylase inhibitors. Data in this panel and subsequent figures are from triplicate experiments. (D) Fatty acid induced EHHADH acetylation and activity. Acetylation and activity of EHHDH ectopically expressed in HEK293T cells were monitored. (E) MDH acetylation. MDH-Myc was expressed in HEK293T cells and acetylation was determined by immunoblotting. (F) Quantitative MS analysis of MDH. FLAG tagged MDH was overexpressed in HEK293T cells and purified by immunoprecipitation. Eluted intact MDH proteins were analyzed by FTICR MS. (G) Glucose enhances MDH acetylation. (H) Activation of MDH by acetylation. The activity of endogenous and ectopically expressed MDH from Chang and HEK293T cells, respectively, were assayed and normalized against actin. (I) Inactivation of MDH by in vitro deacetylation. Immunoprecipitated MDH was incubated with or without CobB deacetylase and activity was assayed. NAD, an essential cofactor for CobB, was omitted as a negative control. (J) Activation of MDH by glucose. Experiments were similar to (H) except cells were treated with glucose.