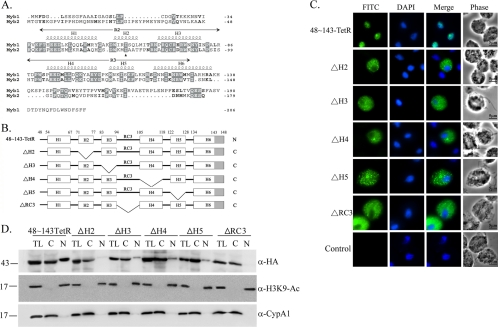
Fig. 6.
Internal deletion to define the essential regions for Myb2 nuclear import. (A) Sequence alignments of Myb1 (AY948338) and Myb2 (AY948337). Secondary structures of aa 40 to 156 of Myb2 and the R2R3 domain of Myb2 are shown on the top of the amino acid sequence. Respective helices with numbers defining their boundaries are indicated as H1 to H6. A random coil connecting H3 and H4 is referred to as RC3. (B) The internal regions in 48∼143TetR were individually deleted as depicted. The gray-shaded area indicates the deleted region of helix 6. C and N indicate the cytoplasmic and nuclear signals, respectively, as examined below. (C) Subcellular localization of 48∼143-TetR, ΔH2, ΔH3, ΔH4, ΔH5, and ΔRC3 was detected by IFA with a mouse anti-HA antibody. The signal was shown as green fluorescence (FITC). The nucleus was stained with DAPI, and cell morphology was recorded by phase-contrast microscopy. Bar, 5 μm. (D) Total lysates (TL) from cells overexpressing 48∼143-TetR, ΔH2, ΔH3, ΔH4, ΔH5, and ΔRC3 were fractionated into the nuclear (N) and cytosolic (C) fractions. Protein samples were separated in 12% gel for Western blotting with the rat anti-HA antibody (right panel). Duplicate blots were examined by the anti-acetyl-histone H3(Lys9) (α-H3K9-Ac) and anti-CypA1 (α-CypA1) antibodies.