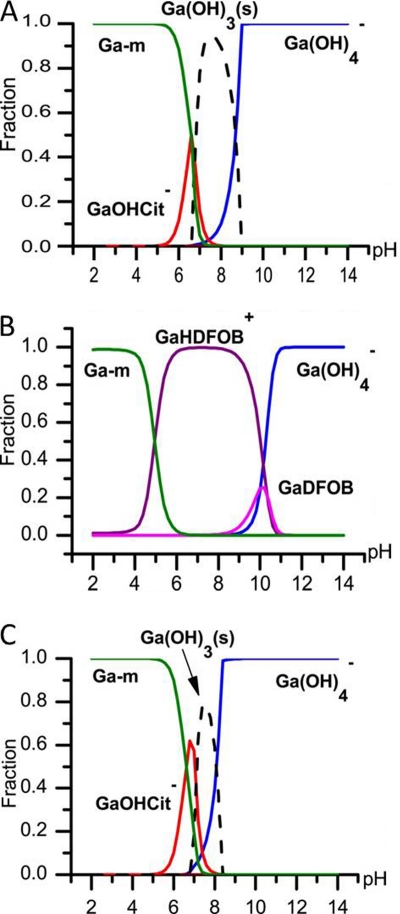
Fig. 1.
Results from the calculation of gallium speciation in 20% Iso-Sensitest medium. Each line represents the fraction of the total amount of gallium in a specific complex in solution. Broken lines represent precipitation of Ga(OH)3. According to the calculations, Ga ions are expected to bind to medium components (Ga-m) only at low pH. At high pH, Ga exists in form of soluble Ga(OH)4−. (A) Twenty micromolar Ga3+ and 50 μM citrate; (B) 20 μM Ga3+ and 20 μM DFOB. (C) At lower total concentrations of Ga3+ (5 μM), the relative precipitation of Ga(OH)3(s) is lower than that at higher concentrations.