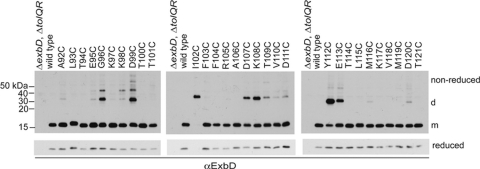
Fig. 1.
Cysteine substitutions in the ExbD periplasmic domain form spontaneous disulfide-linked dimers in vivo. TCA-precipitated proteins from strains expressing chromosomally encoded wild-type ExbD (W3110) or a ΔexbD ΔtolQR strain (RA1045) expressing plasmid-encoded ExbD variants near native ExbD levels (see Table S1 in the supplemental material for induction levels) were resolved on nonreducing or reducing 15% SDS-polyacrylamide gels and immunoblotted with ExbD-specific polyclonal antibodies. Reduced and nonreduced samples came from the same culture. d indicates the position of the homodimer, and m indicates the position of the monomer. The positions of nonreducing molecular mass standards are indicated on the left.