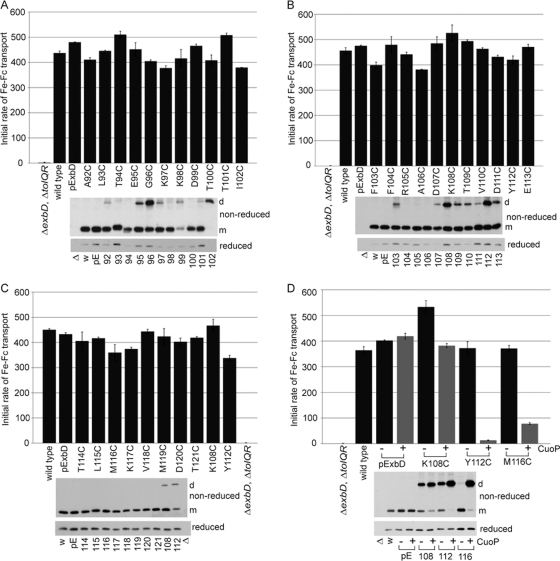
Fig. 2.
ExbD residues 92 through 121 are tolerant of replacement with cysteine, but disulfide-linked dimer formation can inhibit activity. A strain expressing chromosomally encoded wild-type ExbD (W3110) or a ΔexbD ΔtolQR strain (RA1045) expressing plasmid-encoded wild-type ExbD (pExbD) or ExbD Cys substitutions near native ExbD levels (see Table S1 in the supplemental material, 1× M9, for induction levels) was assayed for the ability to support transport of iron-loaded ferrichrome (Fe-Fc) as described in Materials and Methods. The results presented are representative data where activity was within 5% across at least two sets of triplicate assays. The y axis indicates the initial rate of transport. The ExbD variants assayed are indicated along the x axis. TCA-precipitated samples harvested just prior to each assay were resolved on 15% nonreducing or reducing SDS-polyacrylamide gels and immunoblotted with ExbD-specific polyclonal antibody. Δ indicates the ΔexbD ΔtolQR strain (RA1045), w indicates wild-type strain W3110, and pE indicates plasmid-encoded wild-type ExbD. The residue numbers indicate the positions of the ExbD Cys substitution. On the right, d indicates the position of the homodimer, and m indicates the position of the monomer. (A, B, and C) Sets of activity assays performed on the same days. (D) Activity assays for select ExbD homodimers catalyzed by CuoP treatment. − and + indicate buffer only and 0.03 mM CuoP treatment, respectively, prior to assay. The gray bars highlight CuoP-treated strains. The error bars indicate standard errors of the means.