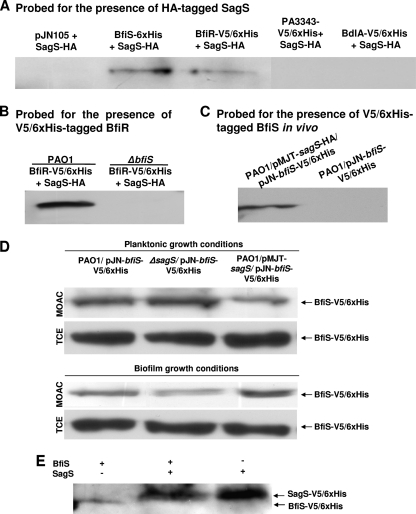
Fig. 7.
SagS directly interacts with and modulates the phosphorylation status of BfiS in a growth-mode-dependent manner. (A) Extracts of PAO1 bearing HA-tagged SagS were incubated with extracts containing 6×His-tagged BfiS or BfiR or a vector control (pJN105), and incubation was followed by anti-6×His immunoprecipitation. The immunoprecipitation eluates were subsequently assessed by immunoblot analysis for the presence of SagS-HA using anti-HA antibodies. Tagged PA3343 and BdlA were used as negative controls. (B) SagS/BfiR interactions in the wild-type (PAO1/pJN-bfiR-V5/6×His, PAO1/pJN-sagS-HA) and ΔbifS mutant (ΔbfiS/pJN-bfiR-V5/6×His, ΔbfiS/pJN-sagS-HA) backgrounds were assessed via anti-HA pulldown assays followed by immunoblot analysis using anti-V5 antibodies for the presence of BfiR-V5/6×His. Cell extracts obtained from PAO1/pJN105 were used as controls. (C) SagS and BfiS form a complex in vivo as demonstrated by pulldown assays using PAO1/pMJT-sagS-HA/pJN-bfiS-V5/6×His. PAO1/pJN-bfiS-V5/6×His was used as a negative control. (D) Phosphorylation of BfiS is modulated by the absence/presence of SagS in a growth-mode-dependent manner, as revealed by anti-V5 immunoblotting for BfiS-V5 in phosphoproteins purified by MOAC and in total cell protein extracts (TCE). (E) Under planktonic conditions, SagS dephosphorylates BfiS.