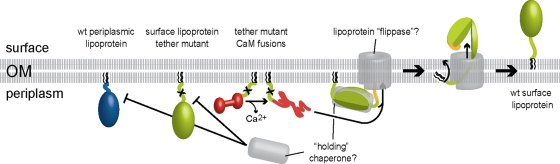
Fig. 6.
Proposed molecular events at the B. burgdorferi OM during surface lipoprotein secretion. Where possible, the color scheme follows that of Fig. 1. Hypothetical components are drawn in gray. Tether mutants of surface lipoproteins such as OspA (light green, with an X marking the tether mutation) fold prematurely in the periplasm, and their secretion through the OM is thereby prevented (17, 18). Periplasmic folding or interactions with periplasmic envelope components may be responsible for subsurface retention of wt periplasmic lipoproteins such as Lp6.6 (blue). Translocation of periplasmic mutant OspA tether-CaM fusion proteins (red) is dependent on chelation-mediated structural changes (this study). Intact surface lipoprotein tether peptides prevent premature folding of proteins—and thereby allow for OM translocation—via interaction with a hypothetical “holding” chaperone (gray). As shown for wt OspA (but also valid for the wt OspA-CaM fusion protein), translocation can initiate at the lipoprotein's C terminus if an unfolded peptide is provided (50; also this study). The peptide portion travels via the hydrophilic lumen of a hypothetical OM lipoprotein “flippase” pore (gray), while the lipid anchor (black) flips from the periplasmic leaflet to the surface leaflet of the OM bilayer; this ultimate anchor topology was recently shown for OspA and B. turicatae Vsp1 (17). In the absence of ATP, the directionality of the process is likely driven by the folding and assembly of the protein on the bacterial surface. This figure updates the Borrelia envelope biogenesis model shown in the work of Bergström and Zückert (7).