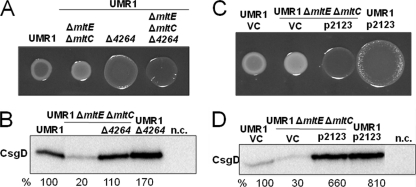
Fig. 5.
Effect of c-di-GMP signaling on the restoration of rdar morphotype expression in the UMR1 ΔmltE ΔmltC double mutant. (A) Deletion of STM4264, encoding a c-di-GMP-dependent phosphodiesterase, in the mltE mltC double mutant upregulated rdar morphotype expression compared to that of the mltE mltC double mutant. As previously shown (45), the STM4264 mutant displayed an upregulated rdar morphotype compared to that of wild-type UMR1. (B) CsgD expression in wild-type UMR1 and mutants. The mltE mltC STM4264 triple mutant shows an increase in CsgD levels compared to that of UMR1 ΔmltE ΔmltC, as CsgD expression increased in S. Typhimurium UMR1 compared to that of its STM4264 mutant. The relative protein levels compared to that of S. Typhimurium UMR1, which was set at 100%, are indicated. n.c., negative-control MAE50 ΔcogD. (C) UMR1 and the mltE mltC double mutant harboring p2123 showed upregulated rdar morphotype expression compared to that of the vector control (VC) pBAD30. (D) CsgD expression in UMR1 and UMR1 ΔmltE ΔmltC containing p2123. CsgD expression increased upon the expression of STM2123 in the wild-type UMR1 and the UMR1 ΔmltE ΔmltC mutant. n.c., negative-control MAE50 ΔcogD. For phenotype observation (A and C), strains were grown on Congo red agar at 28°C for 24 h. To detect CsgD expression (B and D), wild-type UMR1 and mutants were grown on LB without NaCl agar at 28°C for 17 h.