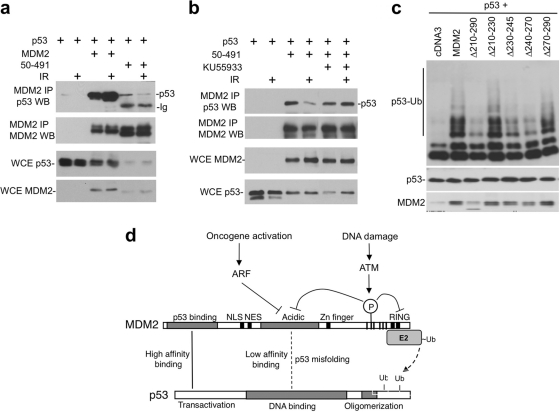
Fig. 10.
ATM inhibits MDM2 acidic domain binding to p53 core. (a) H1299 cells were transfected with p53, MDM2, and MDM2-50-491 for 36 h. Cells were treated with 7 Gy of IR and harvested 4 h later. MDM2 was immunoprecipitated with 2A9 antibody, and the coprecipitated p53 was detected by FL393 Western blotting. (b) The ATM inhibitor KU55933 was used to treat cells during and after IR in an assay similar to that in panel a, showing that the inhibition of MDM2-50-491 and p53 binding by IR was mediated by ATM. (c) H1299 cells were transfected with His6-ubiquitin, p53, and MDM2 acidic domain internal deletion mutants. p53 ubiquitination was analyzed by Ni-NTA pulldown and p53 Western blotting. (d) Model summarizing the mechanism of p53 regulation by ATM. MDM2 bindings to p53 through N-terminal high-affinity interaction, which facilitates a weak second-site interaction between the acidic domain and core domain that activates or positions the RING for ubiquitin transfer to p53. MDM2 RING domain dimerization and oligomerization are also critical for p53 ubiquitination. Phosphorylation by ATM inhibits acidic domain-core binding and RING dimerization, thus blocking two critical steps in p53 ubiquitination. ARF inhibits MDM2 acidic domain function during oncogenic stress, possibly acting through a mechanism similar to phosphorylation.