Abstract
A xylose-inducible gene expression vector for Clostridium perfringens was developed. Plasmid pXCH contains a chromosomal region from Clostridium difficile (xylR-PxylB): xylR, encoding the xylose repressor, xylO, the xyl operator sequence, and PxylB, the divergent promoter upstream of xylBA encoding xylulo kinase and xylose isomerase. pXCH allows tightly regulated expression of the chloramphenicol acetyltransferase reporter and the α-toxin genes in response to the inducer concentration. Thus, pXCH could constitute a new valuable genetic tool for study of C. perfringens.
TEXT
Clostridium perfringens is a Gram-positive anaerobic bacterium that produces potent toxins and histolytic enzymes and causes a variety of diseases, ranging from a mild food-borne diarrheal disease to fulminant and fatal infections, such as dysentery and enterotoxemia in animals and gas gangrene in humans (10, 14). C. perfringens strain 13 is a transformable and extremely poorly sporulating strain with a completely sequenced genome (12). We previously developed a galactose-based in-frame deletion system for genetic studies of strain 13, and we established virulence-attenuated strain HN1314 by disrupting the genes encoding six major secretory proteins, including α-toxin (phospholipase C [PLC]) (7). This strain has been used as a recombinant host to produce clostridial proteins through expression under a strong constitutive promoter (7, 16). Although our expression system is useful for the expression of many clostridial genes, it sometimes fails to produce high levels of recombinant proteins, probably due to the toxicity of the product toward the host cells. To solve such problems, we have attempted to use an inducible promoter system that is lacking in C. perfringens. Such a system would be useful not only to produce high levels of recombinant proteins but also to elucidate the functions of the genes by observing phenotypic changes in depleted constructs and modulated gene expression in the mutants.
In the case of other Firmicutes, a xylose-inducible gene expression system has been developed for Bacillus subtilis (1). The xylAB operon is regulated by XylR. xylR and xylAB are divergently transcribed from a common intergenic region containing the xyl operator (xylO) sequence, which is bound by XylR in the absence of the inducer xylose. Thus, the system basically contains xylR, intergenic xylO, and the xylA promoter. Among clostridia, C. perfringens is unable to utilize xylose, while some other clostridial species are described as xylose-utilizing organisms in Bergey's Manual of Systematic Bacteriology (9). Therefore, we assumed that the xylose-inducible gene expression system could be applied to C. perfringens by introducing an exogenous system from xylose-utilizing clostridia.
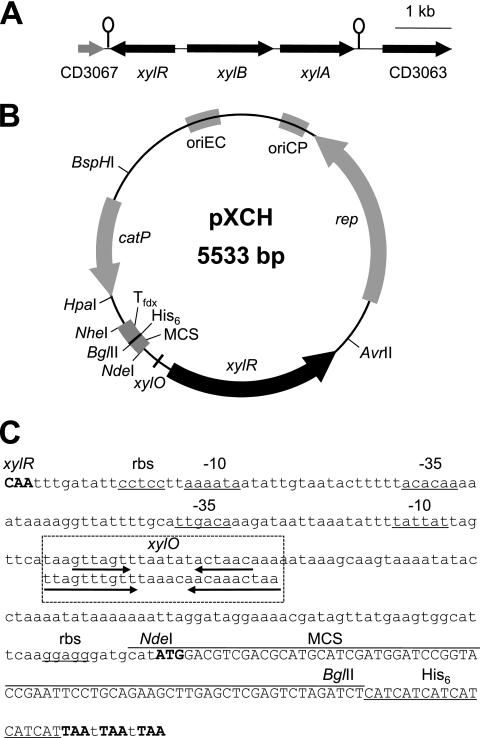
A survey of the xylose metabolism genes in clostridial genomes that have been completely sequenced was carried out using the Pfam (http://pfam.sanger.ac.uk/) information for XylR (PF00480), XylA (PF01261), and XylB (PF00370 and PF02782) in the Comprehensive Microbial Resource (CMR; http://cmr.jcvi.org/cgi-bin/CMR/CmrHomePage.cgi). The survey revealed that C. perfringens does not contain xylose metabolism genes and that C. difficile has divergently located genes of the putative xylR and xylBA operons (Fig. 1 A), in which putative xylO is present upstream of xylBA (Fig. 1B and C). Thus, the C. difficile chromosomal region (xylR-PxylB) from the putative terminator region of xylR to the promoter region of xylB was chosen for construction of xylose-inducible expression plasmid pXCH (Fig. 1B). A PCR-amplified xylR-PxylB region from C. difficile ATCC 9689, obtained with primers Xyl-5 and Xyl-3 (Table 1), was cloned at the AvrII-NdeI sites of Escherichia coli-C. perfringens shuttle vector pCM3 (16), which carries the chloramphenicol resistance gene (catP). The plasmid copy number of pXCH in C. perfringens strain 13 was determined to be 7, based on the copy ratio of the catP to the plc gene (see the supplemental material) and using real-time quantitative PCR (5). We also constructed another xylose-inducible expression plasmid, pXEH (5,761 bp), which contained the erythromycin resistance (ermBP) gene (13). The ermBP gene amplified by primers Em-5 and Em-3 from pFN (15) was replaced with the HpaI-BspHI fragment containing the catP gene in pXCH. All C. perfringens strains constructed were anaerobically grown in TY and TY-G1 medium (7). Chloramphenicol and erythromycin were added to the medium at 10 μg/ml (Cm10) and 20 μg/ml (Em20), respectively, when necessary.
Fig. 1.
(A) Gene organization in the region containing the xyl operon in C. difficile. Genomic information on C. difficile 630 (11) was obtained from the CMR. Stem-loop structures indicate putative rho-independent transcriptional terminators. (B) Schematic representation of xylose-inducible gene expression vector pXCH. rep, oriCP, oriEC, and catP originated from E. coli-C. perfringens shuttle vector pJIR418 (18). Tfdx, the fdx transcriptional terminator region (7); MCS, multiple-cloning site; His6, a six-histidine tag. (C) Intergenic promoter region of the divergently transcribed xylR and xylB genes in pXCH. Translation initiation and stop codons are shown by bold letters. The putative ribosome binding site (rbs) and −10 and −35 sequences are shown as underlined sequences. A putative xylO sequence is aligned with B. subtilis xylO (2) in the box with dashed lines. Palindromic sequences in xylO are indicated by arrows.
Table 1.
Primers used in this study
| Primer | Sequencea |
|---|---|
| Xyl-5 | AAACCTAGGCTAATCTTGCATAAACTATATAATAAATC |
| Xyl-3 | AAACATATGCATCCCTCCTTGAATGCCACTTC |
| PLC-N | AAACATATGAAAAGAAAGATTTGTAAGGCG |
| PLC-C | AAAAGATCTTTATTTTATATTATAAGTTGAATTTCC |
| Em-5 | AAAGTTAACTCCGGAAACGTAAAAGAAGTTATGG |
| Em-3 | AAATCATGAAGCGCTTAGTGGGAATTTGTACC |
| CatP-N | AAACATATGGTATTTGAAAAAATTGATAAAAATAG |
| CatP-C | AAAAGATCTTTAACTATTTATCAATTCCTGCAATTC |
Bold letters indicate the translational initiation or stop codons. The underlined sequences represent restriction enzyme sites.
In order to assess the xylose-inducible expression system we constructed, we cloned catP, which has been used for reporter assays (6), into pXEH. The catP gene amplified by primers CatP-N and CatP-C was cloned at the NdeI-BglII sites of pXEH, resulting in plasmid pX-CatP. Transformant 13/pX-CatP was cultured for 8 h in TY-G1-Em20 containing 0 or 0.03 to 6% xylose. C. perfringens cells were washed three times with ice-cold lysis buffer (25 mM Tris-HCl [pH 7.0], 1 mM EDTA, 250 mM NaCl) and resuspended in the buffer containing 1× protease inhibitor cocktail (Sigma-Aldrich) to an optical density at 600 (OD600) of 20 units/ml. The suspension (200 μl) was incubated at 37°C for 5 min with 1 μg Psm-His, an endolysin for C. perfringens (8). The cells were further disrupted by sonication for 30 s at 4°C. The clear cell lysate obtained on centrifugation at 15,000 × g for 5 min was subjected to the chloramphenicol acetyltransferase (CAT) assay (4). As shown in Fig. 2, the specific CAT activity in the induced cells increased nearly proportionally with the xylose concentration (0.03 to 4% xylose). It should be noted that xylose at less than 4% did not have a significant inhibitory effect on cell growth. Furthermore, no CAT activity was detected under uninduced conditions, indicating this system is tightly regulated in the absence of the inducer. The specific activity of CAT in the cells grown in TY-G1 medium containing 4% xylose was almost the same as that in TY medium containing 4% xylose. This indicated that this system is not affected by glucose, which often causes catabolite repression of the utilization of sugars. It should also be noted that glucose was required for full growth of C. perfringens, and hence TY-G1 medium was used for all subsequent studies.
Fig. 2.
CAT activity in response to the xylose concentration. C. perfringens strain 13 harboring pX-CatP was grown in TY-G1-Em20 (open circles) and TY-Em20 (closed circles) media containing various xylose concentrations (reported as a percentage [wt/vol]). Cultured cells were then subjected to the CAT assay by using clear cell lysates. All assays were performed in triplicate, and the results are expressed as averages. The OD600 of the culture is shown as triangles.
We also examined the system with regard to the production of a secretory protein of C. perfringens. The plc gene amplified by primers PLC-N and PLC-C from pPLC was cloned at the NdeI-BglII sites of pXCH. The resultant plasmid, pX-PLC, was expressed in strain HN1314. HN1314/pX-PLC was grown in TY-G1-Cm10 containing 0 and 0.03 to 4% xylose. As shown in Fig. 3, PLC production in these culture supernatants was examined by means of SDS-PAGE analysis and the egg yolk test (7). PLC production was undetectable in the absence of the inducer but detectable when 0.03% xylose was added to the medium. The level of PLC production increased with increasing concentrations of xylose. This was also the case for the egg yolk test (Fig. 3B). Furthermore, PLC production was not detected in the egg yolk test when HN1314/pX-PLC was grown in the absence of the inducer (Fig. 3C).
Fig. 3.
PLC production in response to xylose concentration. C. perfringens strain 13 harboring pX-PLC was grown in TY-G1-Cm10 containing various xylose concentrations. (A) A 14% SDS-PAGE analysis of the culture supernatants. Xylose concentrations (percentages) in the cultures: 0 (lane 1), 0.03 (lane 2), 0.06 (lane 3), 0.125 (lane 4), 0.25 (lane 5), 0.5 (lane 6), 1 (lane 7), 2 (lane 8), 3 (lane 9), and 4% (lane 10). The OD600 levels in the cultures are shown underneath the gel. (B) PLC activity in the culture supernatants measured on egg yolk (EY)-agarose plates. Fifty-microliter aliquots of the culture supernatants corresponding to the lanes in panel A were applied to wells in EY-TBS agarose plates, followed by incubation for 12 h at 37°C. The medium containing 4% xylose was also applied to the well designated with a C. (C) PLC activity exhibited by colonies on EY-GAM plates. One-microliter aliquots of the cultures were spotted onto EY-GAM plates with (+) or without (−) 4% xylose, followed by a 12-h incubation under anaerobic conditions. (D) Time response to 4% xylose. The cultured cells in TY-G1-Cm10 were resuspended in the same volume of the medium containing 4% xylose and then incubated for 0 (lane 1), 10 (lane 2), 20 (lane 3), 30 (lane 4), 40 (lane 5), 50 (lane 6), and 60 min (lane 7). The culture supernatants were then subjected to 14% SDS-PAGE analysis as described for panel A. Lane 8 is the same sample as in lane 10 of panel A.
We next examined the time response of the system to the inducer. HN1314/pX-PLC was grown in TY-G1-Cm10 for 8 h, and then the harvested cells were resuspended and incubated in an equal volume of TY-G1-Cm10 containing 4% xylose. The PLC production was examined by SDS-PAGE analysis for samples harvested at different incubation times (Fig. 3D). The PLC production was sufficiently high, reaching a maximum within 30 min. These results indicated that the xylose-inducible expression system exhibited a rapid response to the inducer.
In this study, we utilized exogenous elements for the xylose-inducible gene expression system in a xylose-utilizing C. difficile strain. C. difficile also contains the xynBP operon (CD0033-CD0034), which encodes a putative beta-xylosidase and xylose H+-symporter, as in the case of B. subtilis. Although we did not introduce xynP in pXCH, effective xylose-inducible expression was observed in C. perfringens cells that lacked a XynP homologue. This indicates that the elements for xylose-inducible gene expression from C. difficile are functional in C. perfringens and that C. perfringens can take up xylose through an unidentified transport system and/or by simple diffusion.
During the course of this study, a plasmid-based lactose-inducible gene expression system in C. perfringens was reported by Hartman et al. (3). For application of their system, it is preferable to use the mutant strain that they constructed, because the parental strain possesses a lactose-inducible regulatory system. Our system can be used in C. perfringens and other clostridial species, unless they possess xylose-inducible systems. Thus, both systems would be useful as inducible expression vectors, depending on the purposes of a genetic or genetic engineering study. Finally, our genetic system along with an in-frame deletion system (7) could contribute to understanding the pathogenicity and physiology of C. perfringens.
Supplementary Material
Acknowledgments
We thank N. J. Halewood for his assistance in preparing the manuscript.
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (Grant for Scientific Research C 21590482). It was also partly supported by the Kagawa University Characteristic Prior Research Funds 2011.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Bhavsar A. P., Zhao X., Brown E. D. 2001. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl. Environ. Microbiol. 67: 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dahl M. K., Degenkolb J., Hillen W. 1994. Transcription of the xyl operon is controlled in Bacillus subtilis by tandem overlapping operators spaced by four base-pairs. J. Mol. Biol. 243: 413–424 [DOI] [PubMed] [Google Scholar]
- 3. Hartman A. H., Liu H., Melville S. B. 2011. Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens. Appl. Environ. Microbiol. 77: 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaji M., et al. 2003. A novel type of DNA curvature present in a Clostridium perfringens ferredoxin gene: characterization and role in gene expression. Microbiology 149: 3083–3091 [DOI] [PubMed] [Google Scholar]
- 5. Lee C., Kim J., Shin S. G., Hwang S. 2006. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 123: 273–280 [DOI] [PubMed] [Google Scholar]
- 6. Matsushita C., Matsushita O., Koyama M., Okabe A. 1994. A Clostridium perfringens vector for the selection of the promoters. Plasmid 31: 317–319 [DOI] [PubMed] [Google Scholar]
- 7. Nariya H., Miyata S., Suzuki M., Tamai E., Okabe A. 2011. Development and application of a method for counterselectable in-frame deletion in Clostridium perfringens. Appl. Environ. Microbiol. 77: 1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nariya H., et al. 2011. Identification and characterization of a putative endolysin encoded by episomal phage phiSM101 of Clostridium perfringens. Appl. Microbiol. Biotechnol. 90: 1973–1979 [DOI] [PubMed] [Google Scholar]
- 9. Rainey F. A., Hollen B. J., Small A. 2009. Genus 1. Clostridium, p. 738–828 In De Vos P., et al. (ed.), Bergey's manual of systematic bacteriology: Firmicutes, 2nd ed., vol. 3. Springer, New York, NY [Google Scholar]
- 10. Rood J. I. 2007. Clostridium perfringens and histotoxic disease, p. 753–770 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes: a handbook on the biology of bacteria, 3rd ed., vol. 4. Springer, New York, NY [Google Scholar]
- 11. Sebaihia M., et al. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38: 779–786 [DOI] [PubMed] [Google Scholar]
- 12. Shimizu T., et al. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. U. S. A. 99: 996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sloan J., et al. 1992. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 27: 207–219 [DOI] [PubMed] [Google Scholar]
- 14. Songer J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9: 216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamai E., et al. 2008. High-level expression of His-tagged clostridial collagenase in Clostridium perfringens. Appl. Microbiol. Biotechnol. 80: 627–635 [DOI] [PubMed] [Google Scholar]
- 16. Tanaka H., et al. 2011. High-level production and purification of clostripain expressed in a virulence-attenuated strain of Clostridium perfringens. Protein Expr. Purif. 76: 83–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.