Abstract
High-pressure processing (HPP) is a nonthermal technology that has been shown to effectively inactivate a wide range of microorganisms. However, the effectiveness of HPP on inactivation of viruses is relatively less well understood. We systematically investigated the effects of intrinsic (pH) and processing (pressure, time, and temperature) parameters on the pressure inactivation of a nonenveloped virus (human rotavirus [HRV]) and two enveloped viruses (vesicular stomatitis virus [VSV] and avian metapneumovirus [aMPV]). We demonstrated that HPP can efficiently inactivate all tested viruses under optimal conditions, although the pressure susceptibilities and the roles of temperature and pH substantially varied among these viruses regardless of the presence of a viral envelope. We found that VSV was much more stable than most food-borne viruses, whereas aMPV was highly susceptible to HPP. When viruses were held for 2 min under 350 MPa at 4°C, 1.1-log, 3.9-log, and 5.0-log virus reductions were achieved for VSV, HRV, and aMPV, respectively. Both VSV and aMPV were more susceptible to HPP at higher temperature and lower pH. In contrast, HRV was more easily inactivated at higher pH, although temperature did not have a significant impact on inactivation. Furthermore, we demonstrated that the damage of virion structure by disruption of the viral envelope and/or capsid is the primary mechanism underlying HPP-induced viral inactivation. In addition, VSV glycoprotein remained antigenic although VSV was completely inactivated. Taken together, our findings suggest that HPP is a promising technology to eliminate viral contaminants in high-risk foods, water, and other fomites.
INTRODUCTION
High-pressure processing (HPP) is a nonthermal pasteurization technology that uses pressure instead of thermal energy to inactivate harmful pathogens. In the food industry, HPP has been used to inactivate food-borne infectious agents (such as bacteria, viruses, fungi, protozoa, and prions) and proteins (such as enzymes, allergens, and toxins) (2, 3, 6, 9). The primary advantage of HPP is that it has minimal effect on the organoleptic and nutritional properties of foods compared to other processing methods, since the treatment does not disrupt the covalent bonds stabilizing the structure of micronutrients as well as color and flavor compounds (3, 5, 23, 39). In addition, since pressure acts instantaneously and uniformly throughout the pressure vessel, the structure and texture of many high-moisture foods are minimally affected. In today's modern society, consumers are increasingly demanding food products that are minimally processed and free of preservatives. Thus, HPP is becoming a widely used nonthermal processing technology that can ensure food safety, food quality, shelf-life extension, and nutritional value.
Despite the extensive research on the inactivation of bacterial pathogens by HPP, the inactivation of viruses by HPP is less well understood. With regard to applications in the area of food safety, HPP studies have focused on food- and waterborne viruses, including human norovirus, rotavirus, sapovirus, astrovirus, adenovirus, poliovirus, enterovirus 71, and hepatitis A virus (HAV), all of which are nonenveloped viruses (24, 25, 31–35). It is known that these viruses are highly stable in food, water, and other fomites (12, 14, 17, 18, 36, 37). Interestingly, the susceptibilities of food- and waterborne viruses are highly diverse when they are treated with HPP despite their high similarities in virion structure, protein composition, and genetic material (24, 51). Poliovirus, a picornavirus, is extremely resistant to HPP, with less than a 1-log virus reduction achieved after treatment at 600 MPa for 1 h (19, 42, 51). Conversely, HAV, another picornavirus, is quite sensitive to HPP under acidic conditions, with more than a 5-log virus reduction observed after treatment at 350 MPa for 2 min (31–33). Additionally, virus inactivation is dependent upon both process-related parameters (pressure, temperature, and time) and product-related parameters (pH, salt, water activity, and food matrix composition) (31–35, 39). The effect of certain parameters on viral survival is predictable. For example, virus inactivation is enhanced when pressure and holding time are increased (8, 10, 11, 39, 47), and food matrices can in most instances protect virus particles from inactivation (11, 39). However, parameters such as temperature and pH may have more variable or completely opposite roles in inactivating viruses. Murine norovirus 1 (MNV-1), a surrogate for uncultivable human norovirus, was more efficiently inactivated at a cooler temperature (4°C) than at ambient temperature (20°C) (39). In contrast, inactivation of HAV was favored at ambient and higher temperature (31–33). Moreover, MNV-1 was more sensitive to HPP at neutral pH than at acidic pH (39), whereas inactivation of HAV was enhanced at acidic pH (31). Previously, it was shown that 8 log10 50% tissue culture infective doses (TCID50)/ml of rotavirus reduction was achieved after treatment with 300 MPa for 2 min at 25°C (30). In addition, pressure treatment of simian rotavirus SA11 at 250 MPa for 20 min resulted in a 104-fold decrease in the infectivity (45). However, factors influencing pressure inactivation of rotavirus have not been studied. To date, the mechanism by which temperature and pH govern the sensitivity of viruses to HPP is yet to be elucidated.
In comparison with nonenveloped viruses, the sensitivity of enveloped viruses to HPP is less well understood. Although most enveloped viruses are not food borne, a better understanding of the inactivation of enveloped viruses would help to identify the optimal processing parameters, which can facilitate many other important applications of HPP such as preparation of inactivated vaccines. To date, there are only a few studies on high-pressure inactivation of enveloped viruses (20, 22, 27, 41, 49). For example, the titers of herpes simplex virus 1 (HSV-1) and human cytomegalovirus (HCMV) were reduced by more than 7 and 4 logs, respectively, after treatment at 300 MPa at 25°C for 10 min (41). Additionally, a pressure of 260 MPa held for 12 h at 20°C reduced 4 logs of vesicular stomatitis virus (VSV) (49). However, no study that directly compared the pressure sensitivities of nonenveloped and enveloped viruses has been reported. Furthermore, the influence of temperature and pH on the inactivation of enveloped viruses has not been investigated.
The objective of this study is thus to systematically investigate the effects of extrinsic (pressure level, holding time, and treatment temperature) and intrinsic (pH) parameters on the inactivation of a nonenveloped virus (human rotavirus [HRV]) and two enveloped viruses (VSV and avian metapneumovirus [aMPV]) in aqueous media. VSV and aMPV were the two targets of choice because they both belong to nonsegmented negative-sense RNA viruses and share many common characteristics in terms of virion structure, replication, and gene expression strategy.
MATERIALS AND METHODS
Viruses and cell lines.
Human rotavirus (HRV) strain WA (ATCC, Manassas, VA) was propagated in rhesus monkey kidney cells (MA-104), which were grown in Eagle's minimum essential medium (MEM) (ATCC) supplemented with 10% non-heat-inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Confluent monolayers of MA-104 cells were washed with serum-free MEM and infected by HRV at a multiplicity of infection (MOI) of 1. After 1 h of incubation at 37°C under a 5% CO2 atmosphere, 15 ml of Eagle's MEM supplemented with 2% FBS and 6 μg/ml of trypsin (Invitrogen) was added. Virus was harvested after 48 h postinoculation by three freeze-thaw cycles followed by low-speed centrifugation at 1,500 × g for 15 min. Vesicular stomatitis virus (VSV) strain Indiana was a generous gift from Sean Whelan at Harvard Medical School (38), and avian metapneumovirus (aMPV) subtype C strain Minnesota was kindly provided by Mo Saif at The Ohio State University (1). VSV and aMPV were propagated in BHK cells and Vero-E6 cells, respectively. Briefly, both cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% FBS. Confluent cells were infected with VSV and aMPV at MOIs of 0.01 and 1, respectively. Viruses were harvested when extensive cytopathic effect was observed.
Viral plaque assay.
Viral plaque assays were performed as described by Lou et al. with minor modifications (39). HRV plaque assay was performed in MA-104 cells. VSV and aMPV plaque assays were conducted in Vero-E6 cells. Briefly, cells were seeded into six-well plates (BD, Franklin Lakes, NJ) at a density of 2 × 106 cells per well. After 24 h of incubation, cell monolayers were infected with 400 μl of a 10-fold dilution series of each virus, and the plates were incubated for 1 h at 37°C, with agitation every 10 min. The cells were overlaid with 2.5 ml of Eagle minimum essential medium (MEM) containing 1% agarose, 2% FBS, 1% sodium bicarbonate, 0.1 mg of kanamycin/ml, 0.05 mg of gentamicin/ml, 15 mM HEPES (pH 7.7), and 2 mM l-glutamine. Additionally, HRV and aMPV plaque assays were performed in the presence of 2.5 μg/ml trypsin and 1 μg/ml actinomycin D, respectively. Incubation periods for HRV, VSV, and aMPV were 3, 2, and 6 days, respectively. The plates were fixed in 10% formaldehyde, and the plaques were visualized by staining with 0.05% (wt/vol) crystal violet.
Purification of VSV and HRV.
Purification of VSV was performed as described previously (38, 40). Briefly, large stocks of VSV were generated by an infection of 10 confluent T150 flasks of BHK cells. After 24 h postinfection, the cell lysate containing the virus and cell debris was harvested by centrifugation at 1,500 × g for 30 min. Virus was then concentrated by ultracentrifugation in a Ty50.2 rotor (Beckman Coulter, Fullerton, CA) at 40,000 × g for 90 min at 4°C. The pellet was resuspended in 300 μl of NTE buffer (100 mM NaCl, 10 mM Tris, 1 mM EDTA, pH 7.4) and further purified through a 10% (wt/vol) sucrose NTE cushion by centrifugation for 1 h at 150,000 × g at 4°C in an SW50.1 rotor (Beckman). The final VSV-containing pellet was resuspended in 300 μl of NTE buffer overnight. The virus titer and protein content were measured by the plaque assay and Bradford reagent (Sigma Chemical Co.), respectively.
Cell culture-derived rotavirus preparations contain a mixture of double-layered particles (DLPs) and triple-layered particles (TLPs). Purification of HRV TLPs and DLPs was performed as described by Benureau et al (4). Briefly, a large stock of HRV was grown in 8 flasks of confluent MA-104 cells as described above. Virus was harvested by three freeze-thaw cycles, followed by low-speed centrifugation (1,500 × g) for 30 min. The supernatant was purified by ultracentrifugation through a 40% (wt/vol) sucrose cushion for 5 h at 82,000 × g at 4°C in a Ty50.2 rotor (Beckman). TLPs and DLPs were separated by CsCl isopycnic gradient (1.37 g/ml) ultracentrifugation (115,000 × g, 18 h, 4°C, in an SW50.1 rotor). The upper (TLPs) and lower (DLPs) bands were collected separately and suspended in TNC buffer (0.05 M Tris-HCl, 0.15 M NaCl, 15 mM CaCl2, pH 6.5). After centrifugation at 68,000 × g for 2 h, the final TLP- or DLP-containing pellet was resuspended in 300 μl of NTE buffer overnight.
Pressure inactivation of viruses.
One-milliliter aliquots of each virus (106 PFU/ml of HRV and aMPV; 108 PFU/ml of VSV) in cell culture medium were packaged in sterile polyethylene stomacher pouches (Fisher Scientific International, Ontario, Canada) and double sealed in a larger pouch to avoid leakage using an Impulse sealer (American International Electric, Whittier, CA). Pressurization of samples was carried out in a high-pressure unit with temperature control (model Avure PT-1; Avure Technologies, Kent, WA) using water as hydrostatic medium. The virus samples were treated at a range of pressure levels from 200 MPa to 550 MPa at different temperatures (4°C and 20°C) for holding times ranging from 2 min to 10 min. The holding time for all the HPP treatments reported in this study did not include the pressure come-up time (approximately 22 MPa/s) and release time (<4 s). All the temperatures referred to here were the initial sample temperatures. The temperature and pressure during processing were monitored and recorded (Dasytec USA, Bedford, NH). The surviving viruses after processing were quantified by viral plaque assay, and the inactivation kinetics for each virus was determined.
To determine the effect of pH on viral inactivation by HPP, DMEM was artificially adjusted to pH 4.0 and pH 7.0 using citric acid and then filtered with a 0.22-μm membrane filter. The final virus concentration in the pH-adjusted medium was approximately 106 PFU/ml. For HRV and aMPV, samples were pressurized at 250 MPa and 300 MPa at 4°C. For VSV, samples were pressurized at 450 MPa and 550 MPa at 20°C. The holding time for all treatments was 2 min. After pressurization, virus survivors were quantified by plaque assay.
Transmission electron microscopy.
Purified VSV suspensions were treated at 550 MPa and 4°C for 8 min and 10 min, while purified HRV was subjected to 450 MPa at 4°C for 2 min. Twenty microliters of either HPP-treated or untreated samples was fixed in copper grids (Electron Microscopy Sciences, Inc.) and negatively stained with 1% ammonium molybdate. The VSV and HRV particles were visualized with an FEI Tecnai G2 Spirit transmission electron microscope (TEM) operated at 80 kV at the Microscopy and Imaging Facility at The Ohio State University. Images were captured by a MegaView III side-mounted charge-coupled device (CCD) camera (Soft Imaging System, Lakewood, CO).
Analysis of viral proteins by SDS-PAGE.
The proteins of VSV and HRV before and after HPP treatment were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Four microliters of each virus suspension was diluted 5-fold in SDS-PAGE loading buffer containing 1% SDS, 2.5% β-mercaptoethanol, 6.25 mM Tris-HCl (pH 6.8), and 5% glycerol and boiled for 5 min. Viral proteins were separated on a 12% polyacrylamide gel, followed by Coomassie blue staining.
Western blotting.
VSV proteins were separated by 12% SDS-PAGE as described above and further transferred onto a Hybond ECL nitrocellulose membrane (Amersham, Piscataway, NJ) in a Trans-Blot semidry electrophoretic transfer cell (Bio-Rad, Hercules, CA). The blots were blocked in 5% skim milk in PBST (phosphate-buffered saline supplemented with 0.02% Tween) and incubated with mouse monoclonal anti-VSV glycoprotein (G) antibody (Sigma-Aldrich) at a dilution of 1:5,000 in blocking buffer. Afterwards, the blot was incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (1:100,000 dilution). After being washed in PBST for three times (15 min each time), the blots were developed with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Pittsburgh, PA) and exposed to Kodak BioMax MR film (Kodak, Rochester, NY).
RT-PCR.
Purified VSV was treated at 550 MPa and 20°C for 8 min and 10 min, respectively. Purified HRV TLPs and DLPs were treated at 450 MPa at 4°C for 2 min. A viral plaque assay was performed to confirm the complete inactivation of VSV and HRV. The samples were digested with 10 units of RNase at 37°C for 1 h. Viral genomic RNA was extracted from 10 μl of each virus suspension (either HPP treated or untreated) using an RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Reverse transcription-PCR (RT-PCR) was performed using a One Step RT-PCR kit (Qiagen). Based on the sequence of VSV strain Indiana (GenBank accession no. K02033.1), two primers (5′-ATGTCTGTTACAGTCAAGAG-3′ and 5′-TCATTTGTCAAATTCTGAC-3′) were designed to amplify the VSV N gene. Based on the genomic sequence of HRV strain WA (GenBank accession no. FJ423132), two primers (5′-GAGAGAATTTCCGTCTGGCTAA-3′ and 5′-CTTGCCACCACTTTTTCCAAT-3′) were designed to amplify the full-length genomic segment carrying the HRV VP7 gene. The PCR products were analyzed by 1% agarose gel electrophoresis.
Statistical analysis.
All experiments were performed in triplicate. Virus titer was expressed as mean log titer ± standard deviation. Statistical analysis was performed by one-way multiple comparisons using SPSS 8.0 statistical analysis software (SPSS Inc., Chicago, IL). A P value of <0.05 was considered statistically significant.
RESULTS
The effects of temperature and pH on pressure inactivation of HRV.
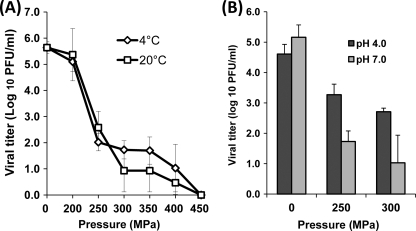
Eagle's MEM was inoculated with HRV at the final concentration of 106 PFU/ml and then processed under different pressures (200 to 450 MPa) and temperatures (4°C and 20°C) for 2 min. As shown in Fig. 1A, similar HRV inactivation kinetics were observed at the two temperatures. The inactivation curves resulting from the pressure treatment appeared quadriphasic, showing (i) a shoulder or an initial lag, (ii) a concave downward slope for the intermediate pressure levels, (iii) a flat tail, and (iv) a second downward slope. HRV was rapidly inactivated in the pressure range of 200 to 300 MPa, reducing the titer to 1 to 2 logs, although viral inactivation became less efficient at pressures ranging from 300 to 450 MPa. Approximately a 5-log virus reduction was observed under 400 MPa at either 4 or 20°C. Viruses were completely inactivated (below the limit of detection by plaque assay) when the pressure increased to 450 MPa for 2 min. Interestingly, temperature did not significantly affect the effectiveness of HRV inactivation throughout the tested pressure range (P > 0.05), although higher temperature (20°C) slightly enhanced HRV inactivation when the pressure level was above 250 MPa. These results demonstrate that (i) HRV can be effectively inactivated by HPP and (ii) temperature plays a minor role in the inactivation of HRV.
Fig. 1.
Effects of temperature and pH on pressure inactivation of HRV in aqueous medium. (A) The effect of temperature on HRV inactivation. HRV stock (106 PFU/ml) in cell culture medium (MEM) was processed under pressures ranging from 200 MPa to 450 MPa held for 2 min at either 4°C or 20°C. (B) The effect of pH on HRV inactivation. HRV stock (105.5 PFU/ml) in MEM was adjusted to pH 4.0 or 7.0 using citric acid. Samples were subjected to pressure treatment at 250 MPa and 300 MPa held for 2 min at 4°C. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations.
The pH value is a critical parameter that affects the effectiveness of pathogen inactivation by HPP (31, 39). Thus, we evaluated the survival of HRV at pH 4.0 and pH 7.0 under two different pressures (250 and 300 MPa). As shown in Fig. 1B, HRV was more easily inactivated at neutral pH than at acidic pH during HPP under both tested pressure levels (P < 0.05). At 250 MPa, 3.4- and 1.2-log virus reductions were observed at pH 7.0 and pH 4.0, respectively. Similarly, at 300 MPa, 4.1 log PFU/ml of HRV lost infectivity at pH 7.0, which was significantly higher than the value at pH 4.0 (1.9 logs) (P < 0.05). Overall, HRV was more sensitive to HPP at neutral pH in aqueous medium.
The effects of temperature and pH on pressure inactivation of VSV.
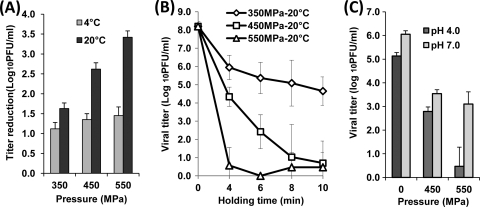
To compare the stabilities of enveloped and nonenveloped viruses to HPP, we evaluated the efficacy of HPP in inactivating VSV, an enveloped virus. Figure 2 represents the survival of VSV after high-pressure treatment. Surprisingly, VSV was much more resistant to HPP than were most of the nonenveloped viruses reported previously (31–35, 39). For example, only 1.1- to 1.5-log virus reductions were achieved for VSV at the pressure range of 350 to 550 MPa for 2 min at 4°C (Fig. 2A). Less than an 0.5-log virus reduction was observed at a pressure level below 350 MPa under the same conditions (data not shown). In contrast, similar treatment conditions under 400 MPa brought more than 5-log virus reductions for MNV-1, feline calicivirus (FCV), HAV, and HRV (31–35, 39). We also evaluated the effect of temperature on inactivation of VSV by HPP. As shown in Fig. 2A, VSV was more easily inactivated at 20°C than at 4°C (P < 0.05). At 550 MPa held for 2 min, 1.5- and 3.4-log reductions of VSV were observed at 4°C and 20°C, respectively. Since VSV is highly resistant to HPP, we increased the HPP holding time to improve the efficacy of VSV inactivation. As shown in Fig. 2B, virus inactivation was enhanced when the holding time was extended. Specifically, approximately a 5-log reduction of VSV was achieved upon treatment at 550 MPa for 4 min. VSV was completely inactivated when HPP holding time was increased to 10 min. Taken together, these results demonstrate that (i) VSV, an enveloped virus, is much more resistant to HPP than are most food-borne viruses (such as HRV and HAV) which are nonenveloped and (ii) VSV is more easily inactivated at 20°C than at 4°C.
Fig. 2.
Effects of temperature, time, and pH on pressure inactivation of VSV in aqueous medium. (A) The effect of temperature on VSV inactivation. VSV stock (108 PFU/ml) in DMEM was treated at 350 MPa, 450 MPa, and 550 MPa for 2 min at either 4°C or 20°C. (B) The effect of pressure holding time on VSV inactivation. VSV was pressure treated at 350 MPa, 450 MPa, and 550 MPa at 20°C for 4, 6, 8, and 10 min. (C) The effect of pH on VSV inactivation. The pH value of medium was adjusted to pH 4.0 or 7.0 using citric acid, and VSV samples were subjected to pressure treatments at 450 MPa and 550 MPa at 20°C for 2 min. The survival of VSV was quantified by plaque assay. Data are the means of three replicates. Error bars represent standard deviations.
Subsequently, we determined the role of pH in VSV inactivation by HPP (Fig. 2C). Briefly, VSV was inoculated into DMEM at pH 4.0 and pH 7.0 and subjected to HPP at two different pressures (450 and 550 MPa). At 450 MPa, there was no significant difference in VSV survival between pH 4.0 and pH 7.0 (P > 0.05). However, at 550 MPa, VSV was more easily inactivated at pH 4.0 (5.7-log reduction) than at pH 7.0 (4.0-log reduction) (P < 0.05). It appears that VSV is more sensitive to HPP at acidic pH than at neutral pH (P < 0.05). However, we also noticed that VSV was unstable in an acidic environment. A 1-log virus reduction was observed when the unpressurized VSV control was incubated in DMEM at pH 4.0 for 24 h at 4°C. Therefore, the virus suspended in an acidic medium could have been more easily sensitized to inactivation at higher pressure.
The effects of temperature and pH on pressure inactivation of aMPV.
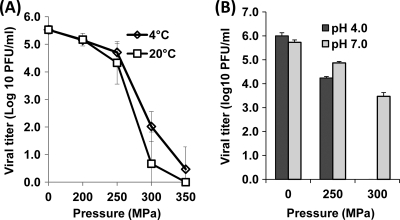
To further investigate the impact of HPP on enveloped viruses, the pressure inactivation kinetics of aMPV, another enveloped virus, was determined. In contrast to VSV, aMPV was found to be quite barosensitive (Fig. 3A). Approximately a 5.1-log virus reduction was achieved upon treatment at 350 MPa at 4°C for 2 min (Fig. 3A), whereas the same treatment reduced VSV by only 1.1 logs (Fig. 2A). Furthermore, inactivation of aMPV was significantly enhanced at 20°C in comparison to 4°C within the pressure range of 200 MPa to 350 MPa (P < 0.05). After treatment at 300 MPa, a 4.9-log reduction was observed at 20°C, while the corresponding reduction at 4°C was about 3.5 logs. Thus, our findings on the pressure inactivation of enveloped viruses suggest that different enveloped viruses have different susceptibilities to HPP, although both VSV and aMPV were more easily inactivated at ambient temperature than at a colder temperature. Overall, the behaviors and responses of different viruses to HPP appear to be independent of the presence of envelopes.
Fig. 3.
Effects of temperature and pH on pressure inactivation of aMPV in aqueous medium. (A) The effect of temperature on aMPV inactivation. aMPV stock (106 PFU/ml) in DMEM was treated at pressures ranging from 200 MPa to 350 MPa at either 4°C or 20°C for 2 min. (B) The effect of pH on aMPV inactivation. aMPV samples at either pH 4.0 or pH 7.0 were processed at 250 MPa and 300 MPa at 4°C for 2 min. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent standard deviations.
The effect of pH on inactivation of aMPV was also determined. As shown in Fig. 3B, aMPV is much more sensitive at pH 4.0 than at pH 7.0 under both 250 MPa and 300 MPa. At the pressure of 300 MPa, aMPV in acidic medium was completely inactivated, while 3.5 logs of virus survivors were still detected after the same treatment at pH 7.0. Moreover, aMPV was considerably more stable in an acidic environment, undergoing no significant reduction in the virus titer at pH 4.0 during incubation of untreated samples (P > 0.05). Taken together, these data support the notion that the combined action of HPP and acidity can deliver a synergistic effect on inactivation of aMPV.
HPP damages virus particles.
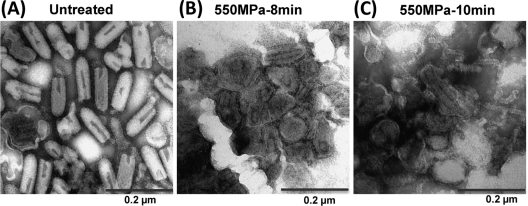
To determine the mechanism of virus inactivation by HPP, we analyzed the pressurized virus particles by electron microscopy (EM). Briefly, highly purified VSV samples (1010 PFU/ml) were treated under 550 MPa at 20°C for 8 min and 10 min separately. While approximately 1 log of VSV remained infectious after 8 min of pressurization, VSV was completely eliminated after 10 min of treatment. The samples were negatively stained with ammonium molybdate, and the virus particles were observed by EM. In the absence of pressurization, the VSV virion is a bullet-shaped particle that is approximately 70 nm in diameter and 140 nm in length (Fig. 4A). After treatment with HPP for 8 min, the morphology of VSV particles was severely distorted. Most virions were round rather than bullet shaped and appeared as dense aggregates. The viral envelope was disrupted and less well defined (Fig. 4B). As expected, more severe damage was observed when the treatment time was extended to 10 min. Indeed, we failed to detect any intact VSV virion after a 10-min treatment (Fig. 4C). The integrity of the viral envelopes was completely lost, and the viral nucleocapsid-genomic RNA (N-RNA) complex leaked out of the damaged particles. In addition, a large amount of debris was observed. These observations indicate that the VSV structure and envelope are completely disrupted by HPP.
Fig. 4.
HPP disrupts VSV particles. Purified VSV was treated by high pressure at 550 MPa at 20°C for 8 min and 10 min separately. Treated and untreated virus particles were negatively stained with 1% ammonium molybdate and visualized by transmission electron microscopy. (A) Untreated VSV virions. (B) VSV particles treated with 550 MPa for 8 min. (C) VSV particles treated with 550 MPa for 10 min.
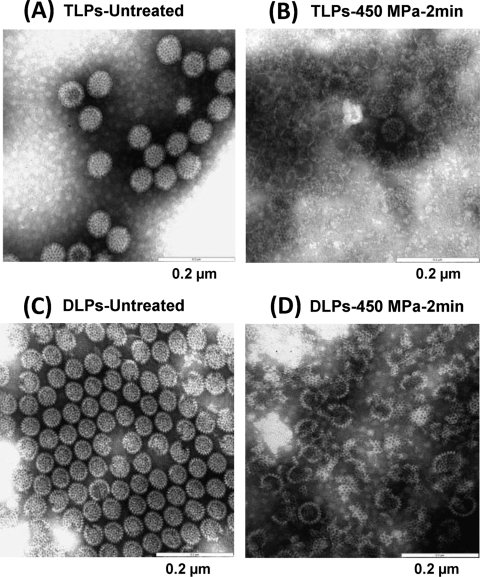
The native infectious rotavirus virions, termed triple-layered particles (TLPs), are composed of three concentric layers of proteins and 11 segments of double-stranded RNA (16). The outermost layer of the virion is comprised of two proteins, VP4 and VP7. VP4 forms dimeric spikes that project from the surface of the virion. The middle layer is comprised of VP6. The innermost layer is composed of three proteins, VP1, VP2, and VP3. During infection, HRV also produces subviral particles, termed the double-layered particles (DLPs), which are composed of two protein capsid layers: an outer layer of VP6 and an inner core of VP, VP2, and VP3 (16). To determine the damage to HRV by HPP, TLPs and DLPs were separated by using CsCl gradient ultracentrifugation. The highly purified TLPs and DLPs were treated at 450 MPa at 4°C for 2 min, and virus particles were visualized by EM. The geometry of intact rotavirus TLPs is wheel shaped with characteristic radiating spokes, smooth edges, and a diameter of approximately 70 to 75 nm (Fig. 5A). After HPP, no intact wheel-like particles were observed. Instead, a large number of DLPs, core particles (single-layered particles), “ring-shaped” particles, and capsid debris were found (Fig. 5B). This result suggests that the outermost and middle layer capsids are disassociated from TLPs and virus particles are disrupted by HPP. The intact DLPs lacking the outer layer are smaller (60 to 65 nm) with rough edges (Fig. 5C). Pressure treatment of DLPs resulted in a large number of broken particles mixed with capsid debris (Fig. 5D). Additionally, some empty particles were observed, indicating possible leakage from the inner core of DLPs after HPP. Taken together, these findings strongly suggest that HPP-induced structural disruption of the virus particles is the underlying mechanism of viral inactivation.
Fig. 5.
HPP disrupts HRV particles. Purified HRV TLPs and DLPs were completely inactivated at 450 MPa at 4°C for 2 min. HPP-treated and untreated samples were negatively stained by 1% ammonium molybdate and visualized by transmission electron microscopy. (A) Untreated HRV TLPs. (B) HRV TLPs treated with 450 MPa for 2 min. (C) Untreated HRV DLPs. (D) HRV DLPs treated with 450 MPa for 2 min.
The effect of HPP on viral proteins.
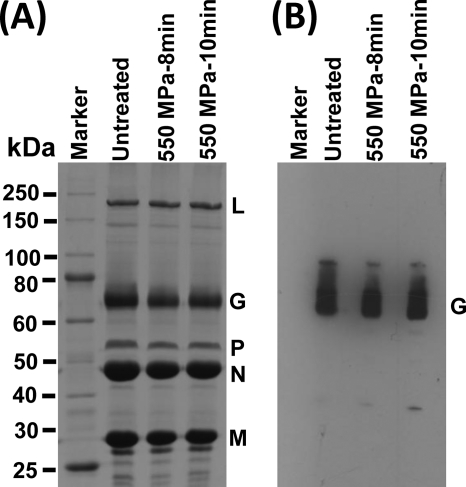
To determine whether HPP disrupted viral structural proteins, we analyzed the pressurized viruses by SDS-PAGE. As shown in Fig. 6A, all five structural proteins of VSV, large (L) polymerase protein, glycoprotein (G), phosphoprotein (P), nucleocapsid (N) protein, and matrix (M) protein, were visualized by SDS-PAGE. The abundances of each viral protein were similar in all untreated and treated samples (P > 0.05), suggesting that even though HPP completely disrupted the virions, the primary or even the secondary structure of viral proteins remained intact upon pressurization. The viral proteins were subjected to Western blotting using a monoclonal antibody against VSV G protein. Comparable amounts of VSV G protein were detected in untreated and treated samples, indicating that G protein remained antigenic upon HPP (Fig. 6B).
Fig. 6.
The effect of HPP on VSV proteins. (A) Visualization of VSV structural proteins by 12% SDS-PAGE. Purified VSV was treated at 550 MPa and 20°C for 8 min and 10 min, respectively. Ten micrograms of total viral proteins was separated by SDS-PAGE followed by Coomassie blue staining. (B) Western blot analysis of VSV G protein. The viral proteins of untreated and treated VSV samples were separated by SDS-PAGE and subjected to Western blotting using a monoclonal anti-VSV G protein.
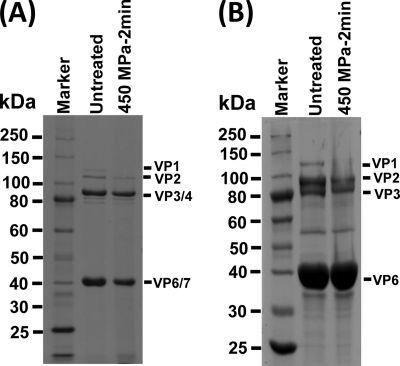
Similarly, we analyzed the effect of HPP on HRV structural proteins using both TLPs and DLPs. As shown in Fig. 7A, all six structural proteins were observed in untreated TLPs, although VP3/VP4 and VP6/VP7 were difficult to separate on a 12% polyacrylamide gel due to their similar molecular weights. However, the abundance of each viral protein was diminished to various degrees after 450-MPa pressure treatment. Particularly, the VP1 protein was undetectable in pressurized TLPs. Similarly, decreased amounts of VP1, VP2, and VP3 were also found in the HPP-treated DLPs (Fig. 7B). VP1 protein was invisible, although 10 μg of total viral proteins was analyzed by SDS-PAGE. Apparently, HPP leads to considerable reduction in the abundance of some viral proteins of HRV.
Fig. 7.
The effect of HPP on HRV proteins. Purified HRV TLPs and DLPs were pressurized at 450 MPa at 4°C for 2 min. The structural proteins of untreated and treated HRV were analyzed by 12% SDS-PAGE followed by Coomassie blue staining. (A) SDS-PAGE of 2 μg of total HRV TLPs. (B) SDS-PAGE of 10 μg of total HRV DLPs.
HPP does not degrade the viral genomic RNA.
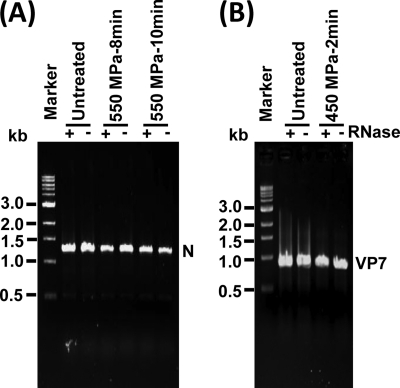
Previously, we reported that HPP did not degrade viral genomic RNA of MNV-1 (39). However, disruption of the capsid of MNV-1 rendered viral genomic RNA sensitive to RNase degradation (39). VSV genomic RNA is strikingly different from that of MNV-1. The RNA genome of MNV-1 is single stranded and positive sense (29). In contrast, the negative-sense RNA genome of VSV is completely encapsidated by the N protein, forming the N-RNA complex (38). To determine whether VSV genomic RNA was released from N protein, we performed an RNase degradation assay. Briefly, the untreated and pressurized VSVs were digested with 10 U of RNase, followed by RNA extraction and RT-PCR to amplify the VSV N gene. The VSV genomic RNA would be degraded by RNase if it had been separated from N protein. As shown in Fig. 8A, a 1.3-kb band corresponding to the VSV N gene was present in all untreated and treated samples regardless of the presence of RNase. The intensities of such bands were essentially identical, indicating that HPP was not able to dissociate VSV genomic RNA from N protein although VSV was completely inactivated at 550 MPa for 10 min at 20°C. For HRV, the 11 segments of double-stranded RNA genome are encapsidated by VP2, which forms the innermost layer of virions. To determine whether RNA genome was released from VP2, HRV was completely inactivated under 450 MPa at 4°C for 2 min, followed by RNase degradation, and the full-length VP7 gene (about 1.1 kb) of HRV was amplified by RT-PCR. As shown in Fig. 8B, the intensities of the VP7 gene amplified from each sample were similar (P > 0.05), demonstrating that the HRV RNA genome was still associated with VP2 protein. Taken together, these findings therefore exclude genomic RNA as a possible target for viral inactivation. In addition, this result also suggests that the structure of VSV N protein and HRV VP2 is highly resistant to HPP.
Fig. 8.
The effect of HPP on viral genomic RNA. (A) The effect of HPP on VSV genomic RNA. Purified VSV was treated at 550 MPa and 20°C for 8 min and 10 min, respectively. The samples were digested with 10 units of RNase at 37°C for 1 h. Viral genomic RNA was extracted from either HPP-treated or untreated VSV. The N gene of VSV was amplified by one-step RT-PCR, and PCR products were visualized by 1% agarose gel electrophoresis. (B) The effect of HPP on HRV genomic RNA. Purified HRV TLPs were treated at 450 MPa at 4°C for 2 min, followed by RNase digestion and RNA extraction. The VP7 gene of HRV was amplified by one-step RT-PCR.
DISCUSSION
To date, the bulk of HPP studies have focused on inactivating food-borne vegetative bacteria, fungi, and protozoa; inactivating viruses by HPP has been relatively understudied. In order to better understand the factors contributing to HPP-induced virus inactivation, we systematically investigated and compared the barosensitivities of one nonenveloped virus (HRV) and two enveloped viruses (VSV and aMPV) under different pressure, temperature, and pH conditions. Overall, all three viruses were efficiently inactivated under optimal processing conditions, and such optimal conditions were specific for each virus, indicating that the pressure threshold and the impacts of temperature and pH on HPP-induced inactivation were unique to each virus. In combination with previous studies in our laboratory as well as those from other groups (7, 8, 24, 25, 31–35, 50), these results provide strong evidence that HPP represents a universal and practical strategy to control the health threat caused by viruses.
Susceptibilities of enveloped and nonenveloped viruses to HPP.
It is generally accepted that an enveloped virus is less stable than a nonenveloped virus since the lipid envelope is susceptible to dryness, pH changes, heat, organic solvents, disinfectants, and many other stresses. In this study, we directly compared the pressure inactivation profiles of enveloped (VSV and aMPV) and nonenveloped (HRV) viruses. We demonstrated that these viruses showed highly different sensitivities in response to HPP regardless of the presence or absence of a viral envelope. While VSV is much more stable than either HRV or aMPV after HPP, the pressure resistance of aMPV is substantially lower than that of HRV. For example, under treatment of 350 MPa at 4°C for 2 min, 1.1-log, 3.9-log, and 5.0-log virus reductions were achieved for VSV, HRV, and aMPV, respectively. Even though VSV and aMPV share similarities in virion structure and other biological properties (1, 15, 38), the structural basis underlying their distinct responses to HPP remains unknown. Of note, pressurization of a few other enveloped viruses, such as avian influenza virus, HSV-1, and HCMV, has also been reported. While treatment under 550 MPa at 15°C for 90 s was able to efficiently inactivate avian influenza virus (H7N7) (27), the titers of HSV-1 and HCMV were reduced by approximately 7 and 4 logs, respectively, upon HPP at more than 400 MPa for 10 min at 25°C (41). Taken together, these results indicate that the susceptibility to pressure varies greatly among enveloped viruses and that VSV is the most stable among tested viruses.
Similarly, nonenveloped viruses also exhibited diverse sensitivities to HPP. Effective viral inactivation (at least a 5-log reduction) resulting from pressure treatment (≤600 MPa) was reported for a variety of nonenveloped viruses, including human norovirus surrogates (feline calicivirus and murine norovirus) (7, 10, 25, 34, 39), HAV (31–33), HRV (30), and coxsackievirus A9 (35). In contrast, the infectivities of Aichi virus and coxsackievirus B5 remained unaffected after 600-MPa treatment at ambient temperature for 5 min (35). Moreover, poliovirus is highly resistant to HPP as evidenced by the fact that less than a 1-log virus reduction was achieved under 600 MPa for 1 h (51). Notably, both coxsackieviruses and poliovirus belong to the genus Enterovirus within the family Picornaviridae, although their sensitivities to high pressure are significantly different from each other.
Collectively, the pressure susceptibilities vary substantially among viruses regardless of the presence of a viral envelope. There is no consensus regarding virus resistance to HPP as judged by the size of the particles or the similarities in virion structure and other biological properties. Also, the sensitivities may not be comparable among viruses within the same genus, family, and/or order. The specific determinants for the sensitivity of a virus to HPP remain to be further elucidated. Perhaps, the sensitivity of a nonenveloped virus may be related to the three-dimensional structure of the viral capsid; thus, differences in the primary amino acid sequences of viral capsid proteins could have a bearing on the virus's resistance to pressure. For enveloped virus, the sensitivity may depend on the stability of the envelope and surface glycoproteins.
Effect of temperature on pressure inactivation of viruses.
Temperature is one of the key factors that affect the efficiency of virus inactivation by HPP. Since all viruses become susceptible to inactivation if a certain critical temperature (56°C) is reached (14), we selected chilling (4°C) and ambient (20°C) temperatures at which viruses are relatively stable. This allows us to determine the effect of pressure on viral survival and not the effect of thermal energy. We found that enveloped viruses, VSV and aMPV, were more easily inactivated at ambient temperature (20°C) across the entire pressure spectrum. Presumably, treatment at higher temperatures could exacerbate pressure-induced destabilization of the lipid envelope, even though further studies are needed to determine whether this phenomenon is observed with other enveloped viruses.
The role of temperature in HPP-induced inactivation of nonenveloped virus appears to be complicated. In the case of HRV, we found that temperature did not significantly influence the effectiveness of inactivation throughout the pressure range. In comparison, previous studies in our laboratory showed that pressure inactivation of MNV-1 is significantly enhanced at refrigeration temperature (4°C) (39). An 8-log reduction of MNV-1 was observed at 400 MPa at 4°C for 2 min, whereas the same treatment at 20°C brought about only a 3-log reduction. Similarly, a 4-min treatment of FCV under 200 MPa at −10°C led to a 5.0-log reduction while the corresponding reduction at 20°C was only 0.3 logs (10). Conversely, inactivation of HAV appears to be favored at ambient temperature (20°C) (31–33). Treatment of HAV under 400 MPa for 1 min at −10°C and 20°C reduced the titers by 1.0 and 2.5 logs, respectively. These observations suggest that temperature plays distinct roles in HPP inactivation of nonenveloped viruses through unknown mechanisms.
Nonetheless, pathogen inactivation is generally enhanced during combined application of pressure and thermal energy (usually above 50°C), by a process termed “temperature-assisted HPP.” For example, HPP (400 MPa) at 50°C for 1 min reduced the titer of HAV by 4.7 logs, whereas similar treatments at −10 and 20°C gave only 1- and 2.5-log reductions, respectively (36). Moreover, treatment of FCV at 200 MPa at 20 and 50°C led to 0.3- and 4.0-log reductions, respectively (10). Similar enhancement has been observed with bacteria and spores upon temperature-assisted HPP (9, 46). Therefore, a combination of pressure and elevated temperature would provide an alternative strategy to inactivate highly resistant pathogens.
Effect of pH on pressure inactivation of viruses.
pH is a critical intrinsic parameter. Like temperature, pH may have a completely opposite effect on virus inactivation by HPP. Here we demonstrated that HRV was more sensitive to HPP at neutral pH than at acidic pH (pH 4.0). After treatment at 250 MPa for 2 min, about 3.4 logs of HRV lost its infectivity at pH 7.0, which was significantly higher than the reduction at pH 4.0 (1.3 logs). Our previous study also showed that inactivation of MNV-1 was substantially enhanced at neutral pH (39). It was also found that inactivation of FCV (7, 10) and tobacco mosaic virus (5) was favored at neutral pH. In sharp contrast, inactivation of HAV was significantly enhanced at pH 4.0 (31). Mechanisms by which pH modulates virus inactivation by HPP are yet to be determined. It is possible that pH affects the stability of the capsid proteins of nonenveloped viruses, thus contributing to HPP-induced inactivation. In the presence of pressure, a virus could be more easily inactivated if its capsid proteins undergo unfavorable structural and biochemical changes at a specific pH. This notion is supported by a study demonstrating that rotavirus spike protein VP4 underwent an irreversible conformational change at elevated pH and that rotavirus with altered VP4 lost its infectivity (44, 45).
As for VSV and aMPV, our results showed that their inactivation by HPP was favored at acidic pH. For instance, 4.6 logs of VSV lost its infectivity at pH 4.0 under 550 MPa, whereas only a 2.9-log reduction was observed at pH 7.0. The effect of pH on aMPV inactivation was more dramatic: 6.0- and 2.2-log virus reductions were achieved at pH 4.0 and pH 7.0, respectively, at 300 MPa for 2 min. It is well known that the viral envelope is highly susceptible to acidic pH, which may contribute to the enhancement in HPP-induced inactivation of enveloped viruses at acidic pH. It is also likely that surface glycoproteins are more easily disassociated from the envelope and/or more easily undergo unfavorable structural changes at acidic pH, thus resulting in loss of viral infectivity.
Mechanism of virus inactivation by HPP.
Our previous studies on MNV-1 showed that the disruption of viral capsid structure, but not the degradation of genomic RNA, was the primary mechanism underlying HPP-induced inactivation (39). To determine whether this is a universal mechanism, we extended our study to VSV and HRV, whose virion structures are strikingly different from those of MNV-1. For VSV, we found that the morphology of its virion was severely altered, the structure and envelope were completely disrupted, and the RNP complex leaked from the particles after HPP. For HRV, the outermost layer of the virion was disrupted and the inner core was released from the particles. For both VSV and HRV, large amounts of debris were observed in the HPP-inactivated viruses. These observations further support the notion that the mechanism underlying HPP-induced virus inactivation is the disruption of the virion structure by breakage of the viral envelope and/or damage to the viral capsid. Because of the limitation of EM, we were unable to determine whether viral glycoproteins dissociated from the envelope or underwent irreversible conformational changes upon HPP. Putatively, any damage to viral glycoproteins could impair virus attachment and entry, which in turn results in the loss of infectivity. Indeed, as discussed below, our results do not rule out the possibility of destabilization of viral glycoproteins by HPP, thus contributing or partially contributing to HPP-induced viral inactivation.
Since HPP has no adverse effect on covalent bonds, it is generally accepted that HPP alters quaternary and tertiary structures, but not primary and secondary structures, of proteins (3, 5, 20, 22, 26). Consistent with this, the abundance of all VSV structural proteins was not significantly altered despite the fact that virion structure was completely disrupted. However, the situation with HRV is somehow different. After HPP, the abundance of each structural protein was reduced to a certain extent, indicating that HRV structural proteins have distinct stabilities under pressure. It is unlikely that these viral proteins were directly degraded by HPP since 450 MPa of pressure should not have a significant impact on primary and secondary structures of a protein. How might we account for this apparent discrepancy? One possibility is that at higher pressure, the structural proteins could be more readily cleaved by nonspecific endogenous proteases. This hypothesis is supported by the fact that efficient infectivity of rotavirus in cell culture requires exogenous trypsin cleavage of spike protein VP4 (4, 13, 16, 43). Routinely, the virus stocks used in our study were grown in the presence of trypsin. Interestingly, it was found that trypsin molecules are tightly associated with the purified virions (4). Trypsin activity is inhibited when associated with virions but is activated upon outer capsid layer solubilization (4). In fact, our mechanistic study demonstrated that HPP led to the disassociation of the outer capsid layer from virions. Thus, it is likely that trypsin activity is activated upon the disruption of the outer capsid layer, which in turn resulted in the degradation of viral structural proteins. It is also important to note that HPP inactivates viruses but often preserves its immunogenic properties. As shown in Fig. 6, even though the VSV virion was completely damaged, its G protein was still recognized by anti-G protein monoclonal antibody. In fact, several studies have shown that pressurized virus particles retained good immunogenicity in animal models (21, 28, 48).
In conclusion, HPP is capable of efficiently inactivating common enveloped and nonenveloped viruses although they have various pressure susceptibilities and require different optimal conditions for efficient inactivation. Moreover, disruption of the virion structure, viral capsid, and envelope, but not the degradation of viral genomic RNA, is the primary mechanism of viral inactivation by HPP. HPP is thus a promising technology to eliminate various viral contaminants in high-risk foods and water. In addition, HPP may be a promising method to generate effective vaccines since it inactivates viruses without losing the immunogenicity of specific viral proteins.
ACKNOWLEDGMENTS
This study was supported by a special emphasis grant (2010-01498) from the USDA National Integrated Food Safety Initiative (NIFSI) and a food safety challenge grant (2011-68003-30005) from the USDA Agriculture and Food Research Initiative (AFRI) to J.L. and H.C.
We thank Sean Whelan for VSV and Mo Saif for aMPV. We thank members of the Li laboratory for critical reviews of the manuscript.
Footnotes
Published ahead of print on 14 October 2011.
REFERENCES
- 1. Alkhalaf A. N., Ward L. A., Dearth R. N., Saif Y. M. 2002. Pathogenicity, transmissibility, and tissue distribution of avian pneumovirus in turkey poults. Avian Dis. 46:650–659 [DOI] [PubMed] [Google Scholar]
- 2. Baert L., Debevere J., Uyttendaele M. 2009. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. Food Microbiol. 131:83–94 [DOI] [PubMed] [Google Scholar]
- 3. Balny C., Masson P., Heremans K. 2002. High pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1595:3–10 [DOI] [PubMed] [Google Scholar]
- 4. Benureau Y., Huet J. C., Charpilienne A., Poncet D., Cohen J. 2005. Trypsin is associated with the rotavirus capsid and is activated by solubilization of outer capsid proteins. J. Gen. Virol. 86:3143–3151 [DOI] [PubMed] [Google Scholar]
- 5. Bonafe C. F., et al. 1998. Tobacco mosaic virus disassembly by high hydrostatic pressure in combination with urea and low temperature. Biochemistry 37:11097–11105 [DOI] [PubMed] [Google Scholar]
- 6. Brown P., Meyer R., Cardone F., Pocchiari M. 2003. Ultra-high-pressure inactivation of prion infectivity in processed meat: a practical method to prevent human infection. Proc. Natl. Acad. Sci. U. S. A. 100:6093–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buckow R., Isbarn S., Knorr D., Heinz V., Lehmacher A. 2008. Predictive model for inactivation of feline calicivirus, a norovirus surrogate, by heat and high hydrostatic pressure. Appl. Environ. Microbiol. 74:1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calci K. R., Meade G. K., Tezloff R. C., Kingsley D. H. 2005. High-pressure inactivation of hepatitis A virus within oysters. Appl. Environ. Microbiol. 71:339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H., Hoover D. G. 2003. Pressure inactivation kinetics of Yersinia enterocolitica ATCC 35669. Int. J. Food Microbiol. 87:161–171 [DOI] [PubMed] [Google Scholar]
- 10. Chen H., Hoover D. G., Kingsley D. H. 2005. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 68:2389–2394 [DOI] [PubMed] [Google Scholar]
- 11. Chen H., Guan D., Hoover D. G. 2006. Sensitivities of foodborne pathogens to pressure changes. J. Food Prot. 69:130–136 [DOI] [PubMed] [Google Scholar]
- 12. Cliver D. O. 1997. Virus transmission via food. Food Technol. 51:71–78 [PubMed] [Google Scholar]
- 13. Dormitzer P. R., Greenberg H. B., Harrison S. C. 2001. Proteolysis of monomeric recombinant rotavirus VP4 yields an oligomeric VP5* core. J. Virol. 75:7339–7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duizer E., et al. 2004. Inactivation of caliciviruses. Appl. Environ. Microbiol. 70:4538–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Easton A. J., Domachowske J. B., Rosenberg H. F. 2004. Animal pneumoviruses: molecular genetics and pathogenesis. Clin. Microbiol. Rev. 17:390–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estes M. K., Cohen J. 1989. Rotavirus gene structure and function. Microbiol. Rev. 53:410–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estes M. K., Prasad B. V., Atmar R. L. 2006. Noroviruses everywhere: has something changed? Curr. Opin. Infect. Dis. 19:467–474 [DOI] [PubMed] [Google Scholar]
- 18. Feng K., Divers E., Ma Y., Li J. 2011. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl. Environ. Microbiol. 77:3507–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferreira E., et al. 2009. Effects of hydrostatic pressure on the stability and thermostability of poliovirus: a new method for vaccine preservation. Vaccine 27:5332–5337 [DOI] [PubMed] [Google Scholar]
- 20. Gaspar L. P., et al. 2002. Hydrostatic pressure induces the fusion-active state of enveloped viruses. J. Biol. Chem. 277:8433–8439 [DOI] [PubMed] [Google Scholar]
- 21. Gogal R. M., Jr., et al. 2011. High hydrostatic pressure processing of murine norovirus 1-contaminated oysters inhibits oral infection in STAT-1(-/-)-deficient female mice. J. Food Prot. 74:209–214 [DOI] [PubMed] [Google Scholar]
- 22. Gomes A. M., Pinheiro A. S., Bonafe C. F., Silva J. L. 2003. Pressure-induced fusogenic conformation of vesicular stomatitis virus glycoprotein. Biochemistry 42:5540–5546 [DOI] [PubMed] [Google Scholar]
- 23. Gross M., Jaenicke R. 1994. Proteins under pressure: the influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur. J. Biochem. 221:617–630 [DOI] [PubMed] [Google Scholar]
- 24. Grove S. F., et al. 2006. Inactivation of foodborne viruses of significance by high pressure and other processes. J. Food Prot. 69:957–968 [DOI] [PubMed] [Google Scholar]
- 25. Grove S. F., et al. 2008. Inactivation of hepatitis A virus, poliovirus and a norovirus surrogate by high pressure processing. Innov. Food Sci. Emerg. Technol. 9:206–210 [Google Scholar]
- 26. Heremans R., Smeller L. 1998. Protein structure and dynamics at high pressure. Biochim. Biophys. Acta 1386:353–370 [DOI] [PubMed] [Google Scholar]
- 27. Isbarn S., Buckow R., Himmelreich A., Lehmacher A., Heinz V. 2007. Inactivation of avian influenza virus by heat and high hydrostatic pressure. J. Food Prot. 70:667–673 [DOI] [PubMed] [Google Scholar]
- 28. Ishimaru D., Sá-Carvalho D., Silva J. L. 2004. Pressure-inactivated FMDV: a potential vaccine. Vaccine 22:2334–2339 [DOI] [PubMed] [Google Scholar]
- 29. Karst S. M., Wobus C. E., Lay M., Davidson J., Virgin H. W., IV 2003. STAT 1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578 [DOI] [PubMed] [Google Scholar]
- 30. Khadre M. A., Yousef A. E. 2002. Susceptibility of human rotavirus to ozone, high pressure, and pulsed electric field. J. Food Prot. 65:1441–1446 [DOI] [PubMed] [Google Scholar]
- 31. Kingsley D. H., Chen H. 2009. Influence of pH, salt, and temperature on pressure inactivation of hepatitis A virus. Int. J. Food Microbiol. 130:61–64 [DOI] [PubMed] [Google Scholar]
- 32. Kingsley D. H., Guan D., Hoover D. G. 2005. Pressure inactivation of hepatitis A virus in strawberry puree and sliced green onions. J. Food Prot. 68:1748–1751 [DOI] [PubMed] [Google Scholar]
- 33. Kingsley D. H., Hoover D., Papafragkou E., Richards G. P. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 65:1605–1609 [DOI] [PubMed] [Google Scholar]
- 34. Kingsley D. H., Holliman D. R., Calci K. R., Chen H., Flick G. J. 2007. Inactivation of a norovirus by high-pressure processing. Appl. Environ. Microbiol. 73:581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kingsley D. H., Chen H., Hoover D. 2004. Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Res. 102:221–224 [DOI] [PubMed] [Google Scholar]
- 36. Kingsley D. H., Guan D., Hoover D. G., Chen H. 2006. Inactivation of hepatitis A virus by high-pressure processing: the role of temperature and pressure oscillation. J. Food Prot. 69:2454–2459 [DOI] [PubMed] [Google Scholar]
- 37. Leon J. S., et al. 2011. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl. Environ. Microbiol. 77:5476–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J., Fontaine-Rodriguez E. C., Whelan S. P. 2005. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J. Virol. 79:13373–13384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lou F., Neetoo H., Chen H., Li J. 2011. Inactivation of a human norovirus surrogate by high-pressure processing: effectiveness, mechanism, and potential application in the fresh produce industry. Appl. Environ. Microbiol. 77:1862–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma Y., Li J. 2011. Vesicular stomatitis virus as a vector to deliver human norovirus virus-like particles: a new vaccine candidate against an important non-cultivable virus. J. Virol. 85:2942–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakagami T., et al. 1992. Inactivation of herpes viruses by high hydrostatic pressure. J. Virol. Methods 38:255–261 [DOI] [PubMed] [Google Scholar]
- 42. Oliveira A. C., et al. 1999. Low temperature and pressure stability of picornaviruses: implication for virus uncoating. Biophys. J. 76:1270–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parashar U. D., Hummelman E. G., Bresee J. S., Miller M. A., Glass R. I. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pesavento J. B., Crawford S. E., Roberts E., Estes M. K., Prasad B. V. 2005. pH-induced conformational change of the rotavirus VP4 spike: implications for cell entry and antibody neutralization. J. Virol. 79:8572–8580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pontes L., et al. 2001. Pressure-induced formation of inactive triple-shelled rotavirus particles is associated with changes in the spike protein VP4. J. Mol. Biol. 307:1171–1179 [DOI] [PubMed] [Google Scholar]
- 46. Reineke K., Mathys A., Knorr D. 2011. The impact of high pressure and temperature on bacterial spores: inactivation mechanisms of Bacillus subtilis above 500 MPa. J. Food Sci. 76:189–197 [DOI] [PubMed] [Google Scholar]
- 47. Sánchez G., Aznar R., Martínez A., Rodrigo D. 2011. Inactivation of human and murine norovirus by high-pressure processing. Foodborne Pathog. Dis. 8:249–253 [DOI] [PubMed] [Google Scholar]
- 48. Shearer A. E., Kniel K. E. 2009. High hydrostatic pressure for development of vaccines. J. Food Prot. 72:1500–1508 [DOI] [PubMed] [Google Scholar]
- 49. Silva J. L., Luan P., Glaser M., Voss E. W., Weber G. 1992. Effects of hydrostatic pressure on a membrane-enveloped virus: high immunogenicity of the pressure-inactivated virus. J. Virol. 66:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang Q., et al. 2010. Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int. J. Food Microbiol. 137:186–189 [DOI] [PubMed] [Google Scholar]
- 51. Wilkinson N., Kurdzeil A. S., Langton S., Needs E., Cook N. 2001. Resistance of poliovirus to inactivation by high hydrostatic pressures. Innov. Food Sci. Emerg. Technol. 2:95–98 [Google Scholar]