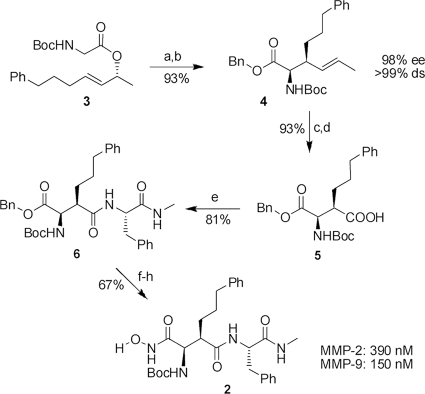
Fig. 4.
Synthesis and evaluation of MMP inhibitor 2. The reagents and conditions were as follows. (a) 1.2 eq ZnCl2, 2.5 eq lithium diisopropylamide (LDA), tetrahydrofurane (THF), −78°C to room temperature (RT), 20 h. (b) 1.1 eq Cs2CO3, 3.5 eq benzyl bromide (BnBr), dimethylformamide (DMF), 0°C to RT, 20 h. (c) 1, O3, CH2Cl2, −78°C, 5 min; 2, PPh3, −78°C, 30 min. (d) 10 eq NaClO2, 7 eq NaH2PO4 · 2H2O, 20 eq 2-methyl-2-butene, t-butanol/H2O, RT, 16 h. (e) 1.0 eq 2-(1H-benzotriazole-1-yl-1,1,3,3-tetramethylaminum tetrafluoroborate (TBTU), 1.0 eq NEt3, 1.2 eq (S)-PhNHMe, CH2Cl2, 0°C to RT, 18 h. (f) Pd/C (10%), H2, THF (90%). (g) 1.0 eq ClCOOiBu, 1.0 eq NEt3, 2.0 eq O-benzylhydroxylamine, THF, −20°C to RT, 18 h (90%). (h) Pd/C (10%), H2, DMF (83%). ee, enantiomeric excess; ds, diastereoselectivity.