Abstract
Objectives
According to ancient Chinese medicine, kudzu root has been used as an ingredient to treat alcohol intoxication for centuries. Kudzu root extract is effective at reducing alcohol intake in animals and in humans, both in a natural-settings laboratory environment and on an outpatient basis. In dependent populations, withdrawal from alcohol is associated with disturbed sleep. These disturbances to the quantity and quality of sleep likely impact relapse to drinking. Many medications used to treat alcohol dependence also affect sleep. Therefore, as a possible treatment for alcohol dependence, the impact of kudzu root extract on the sleep/wake cycle is an important aspect of its effectiveness.
Design
This double-blind, placebo-controlled, crossover trial tested the effects of kudzu root extract on the sleep/wake cycles of moderate drinkers.
Results
Kudzu extract had no effect on any of the sleep parameters measured, including sleep efficiency, sleep latency, total time asleep per night, number of waking episodes, time awake per episode, number of moving minutes, number of sleep episodes, time asleep per episode, and number of immobile minutes.
Conclusions
These data suggest that the administration of kudzu root extract does not disturb sleep/wake cycles of moderate drinkers, and as such its utility as an adjunct treatment for alcohol dependence remains free of any potential side-effects on sleep.
Introduction
The Chinese medication (translated into English from the original Chinese characters as XJL), which contains a combination of Pueraria lobota (kudzu root), Citrus reticulate (mandarin orange peel), Schisandra chinensis (five flavor berry), Hovenia dulcis (Japanese raisin tree), Panax ginseng (ginseng), vitamin C, and sugar, has been utilized as a treatment for alcohol intoxication since at least 600 ad (Li SC, Ben Cho Gang Mu, 1603; Sun S-M, Beiji-Quianjin-Yaofang, circa 600 ad). One of the main ingredients in the original Chinese medication is kudzu root (P. lobata), a member of the pea family that grows in the southern United States and Asia. More recently, a kudzu root extract preparation (NPI-028; Natural Pharmacia International [NPI] preparation #28) has been shown to reduce alcohol intake in several strains of alcohol-preferring rats1 and monkeys.2 Kudzu root contains three main isoflavones that decrease alcohol consumption in animal models: daidzin (NPI-031D), daidzein (NPI-031E), and puerarin (NPI-031G). Treatment with either daidzin or daidzein reduces alcohol intake in Syrian Golden hamsters, with alcohol intake increasing back toward baseline levels after treatment cessation.3,4 Injection of daidzin,5 and oral administration of daidzin, daidzein, or puerarin reduces alcohol intake in rats, with consumption again increasing back toward baseline after treatment cessation.6
Kudzu root extract has been available for human consumption in an over-the-counter preparation for a number of years; however, the Food and Drug Administration does not regulate herbal medications and nutritional supplements. Thus, the extract preparations of commercially available kudzu root vary widely in their isoflavone concentrations, and ingestion of different types of supplements results in varying concentrations of isoflavones in plasma.7 To ensure homogeneity in isoflavone concentration, a standardized kudzu root extract preparation was developed (NPI-031, Natural Pharmacia International [NPI], Burlington, MA). This preparation of kudzu root extract is derived from a powder containing the entire root, which is then standardized to contain 125 mg total isoflavones (19% puerarin, 4% daidzin, and 2% daidzein) in each 500-mg capsule.8
In a double-blind, placebo-controlled, crossover trial, binge drinkers received this kudzu root extract preparation (two 500-mg capsules with 125-mg isoflavones each, taken t.i.d.) or placebo for 1 week, in counterbalanced order.9 Participants were then brought into a natural settings laboratory for a 1.5-hour drinking session during which they had free access to up to 6 beers. Following kudzu root extract, but not placebo treatment, participants drank fewer beers, with an increased number of sips per beer, and a decrease in the amount consumed per sip, as well as an increased latency to open each beer.9 Results in a laboratory or hospital environment are not always consistent with behaviors when participants return to their home environments, which contain all of the cues that can trigger drinking. Therefore, Lukas et al. then administered kudzu root extract to heavy drinkers who recorded their alcohol consumption while going about their daily lives. Even in this outpatient study design, participants who received kudzu root extract reported significantly less alcohol consumption than participants who received placebo (Lukas et al., unpublished data). Interestingly, in both studies this reduction in alcohol consumption occurred without any effect on craving.9
In dependent populations, decreasing alcohol consumption is often associated with insomnia and disturbances to sleep quality and quantity.10,11 A number of medications used to treat alcohol dependence differentially impact sleep. For instance, clomethiozole exacerbates withdrawal-associated sleep disturbances,12 while lorazepam ameliorates these disturbances to sleep.13 Acamprosate lessens sleep disturbances in a dependent population,14 even while it has no effect on sleep in healthy participants.15 Results with gabapentin are mixed, with some reporting an improvement in disturbed sleep,13 and others reporting no effect on sleep.16 Normalization of sleep during alcohol withdrawal is important because these withdrawal-associated sleep disturbances may precipitate or contribute to relapse.13,17,18
Any effect kudzu root extract may have on sleep is an important consideration for administration of this preparation as a potential medication to treat alcohol dependence. This double-blind, placebo-controlled crossover trial tested the effects of kudzu root extract on the sleep/wake cycles of a population who continued to drink in their normal pattern, and thus were not experiencing withdrawal-related sleep disturbances. These participants were moderate drinkers and were not attempting to decrease alcohol intake, thus enabling assessment of the impact of kudzu root extract alone on sleep quality and quantity.
Materials and Methods
Participants
Ten (10) healthy adult (age 28.5±6.84 years) moderate-drinking (7.55±1.92 drinks/week) men (n=6) and women (n=4) took part in each of three phases (kudzu root extract; 750 mg/day total isoflavones), placebo (gelatin capsules, 650 mg, Nature's Bounty, Inc., Bohemia, NY), and an intervening no-treatment period. Two (2) participants were excluded from the final analyses for not following study procedures (repeatedly removing the watch during sleep) or for lack of data due to device malfunction.
All participants were recruited following approval by the McLean Hospital Institutional Review Board (IRB). Moderate drinkers were recruited from the community through advertisements in local and college newspapers as well as advertisements posted on bulletin boards in the Boston area. Responders who passed an initial phone screen visited the Behavioral Psychopharmacology Research Laboratory at McLean Hospital, where they read and signed an IRB-approved informed consent form and passed a physical (electrocardiogram, complete blood panel including a test of liver function, drug urine screen [QuickTox Drug Screen Dipcard, Branan Medical Corporation, Irvine, CA], and pregnancy tests for females [Stanbio QuPID Procedure No. 1220, Studio Laboratory, Boerne, Texas]). They also were assessed for, and were free of, any Axis I disorders Structured Clinical Interview for DSM-IV disorders,19 including drug and alcohol use disorders.
Experimental procedures
Participants who met requirements during the initial screening visit were then fitted with a wrist actigraphy device (ActiWatch-Score, Mini-Mitter/Respironics, Bend, OR), which is worn similarly to a wrist watch and on which participants could input substance use and craving by pushing a button on the face of the “watch” using the following guide: “1” for Alcohol, “2” for Tobacco, “3” for Caffeine, “4” for Cocaine, “5” for Marijuana, “6” for Medication (kudzu root extract or placebo), “7” for Ecstasy, “8” for Hallucinogens, “9” for Opiates, “10” for Other. Craving was assessed every 3 hours±20 minutes, at which time the watch beeped and participants were asked to press the button in order to record their subjective craving for alcohol at that moment on a scale of 1 for “not at all” to 10 for “extremely.”
For 9 days participants took placebo or kudzu root extract (two 500-mg capsules, each containing 125 mg total isoflavones 3 times a day, for a total isoflavone concentration of 750 mg/day; NPI-031; Alkontrol-Herbal,® NPI) in counterbalanced order with an intervening no-treatment washout period of approximately 21 days. Along with morning and evening doses of medication or placebo, participants took 25 mg of riboflavin (vitamin B2), which causes fluorescence of urine when it is exposed to UV light. Medication compliance was determined by repeated monitoring of urinary fluorescence20 as well as daily diary reports of pill taking.
Analysis
The ActiWatch-Score wrist actigraphy device contains an accelerometer that records instances and magnitudes of movements. To calculate sleep variables, Acti-Ware Sleep software (Mini-Mitter/Respironics, Bend, OR) uses an algorithm designed to take into account the magnitude of movement immediately surrounding the epoch in question. In this way, the software can differentiate between a pattern of movement that constitutes a waking episode versus a pattern of movement that constitutes a simple shift in body position during sleep (Mini Mitter Corporation21). Bedtime and rise time were entered from participants' daily diaries. Acti-Ware software uses these times paired with accelerometer data collected via the ActiWatch device to calculate sleep parameters. Bedtimes were missing or faulty for 3/148 (2%) nights, so sleep latency was determined from actigraphy data alone for those nights. Outliers were defined as values more than 3 standard deviations from the mean and were discarded from the analysis. The total numbers of outliers per dependent measure were as follows: sleep efficiency–3, sleep latency–3, total sleep time–2, wake episodes–1, time awake per episode–3, moving minutes–2, sleep episodes–2, time asleep per episode–4, and immobile minutes–2. All data are expressed as average±standard deviation.
In order to assess the impact of kudzu root extract on sleep parameters, alcohol use, and alcohol craving, Mixed Model analyses of variance were run in SPSS (version #18, SPSS Inc., Chicago, IL) with the sleep parameter (sleep efficiency, sleep latency, wake after sleep onset, number of wake episodes, time awake per episode, number of sleep episodes, time asleep per episode, number of immobile minutes, alcohol use, and alcohol craving) as the dependent variable, medication phase (kudzu root extract, or placebo) as the fixed factor main effect, and participant as the random effect. The Bonferroni method was used to correct for multiple comparisons.
Results
Sleep
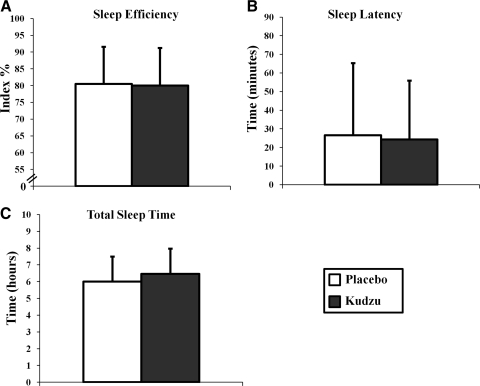
In moderate drinkers, kudzu root extract treatment had no effect on standard parameters of sleep. Sleep efficiency, a measure of the time spent asleep in relation to the time spent in bed, was no different during placebo or kudzu root extract treatment phases (Fig. 1A; F(1,143)=0.059; p=0.808). Sleep latency, the amount of time between when an individual gets into bed, and when he/she falls asleep, was also similar in both phases of the trial (Fig. 1B; F(1,143)=0.161, p=0.689). Finally, total sleep time per night was no different during either phase (Fig. 1C; F(1,144)=0.110, p=0.741). These data indicate that 9 days of kudzu root extract treatment does not affect any of the standard parameters of sleep in this moderately drinking population.
FIG. 1.
Effects of kudzu root extract on sleep efficiency (A: kudzu, n=75 nights; placebo, n=70 nights), sleep latency (B: kudzu, n=74 nights; placebo, n=71 nights), and the total time asleep per night (C: kudzu, n=75 nights; placebo, n=71 nights). Data are graphed as averages±standard deviations.
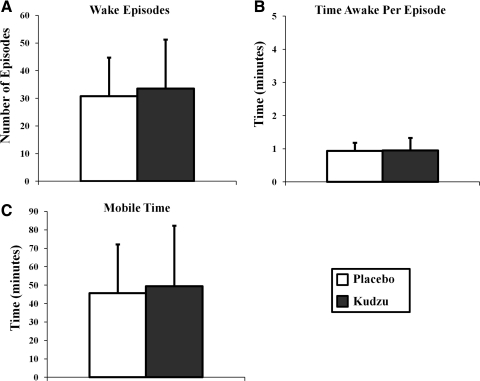
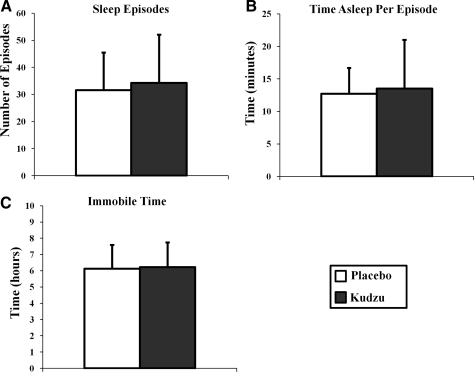
Kudzu root extract treatment had no effect on measures of night-time wakefulness and sleep quality and quantity. The number of waking episodes during the night (Fig. 2A; F(1,145)=1.039, p=0.310), the amount of time spent awake per episode (Fig. 2B; F(1,141)=0.058, p=0.810), and the time spent mobile during the night (Fig. 2C; F(1,144)=0.569, p=0.452) were not different during the placebo or kudzu root extract treatment phases. Similarly, the number of sleep episodes during the night (Fig. 3A; F(1,145)=1.008, p=0.317), the amount of time spent asleep per episode (Fig. 3B; F(1,140)=0.667, p=0.415), and the time spent immobile (Fig. 3C; F(1,144)=0.140, p=0.709) were similar during both study phases. Thus, in this moderately drinking population, every aspect of sleep quality and quantity measured was not affected by 9 days of kudzu root extract treatment.
FIG. 2.
Effects of kudzu root extract on the number of waking episodes (A: kudzu, n=76 nights; placebo, n=71 nights), time awake per episode (B: kudzu, n=75 nights; placebo, n=68 nights), and the number of minutes moving (C: kudzu, n=75 nights; placebo, n=71 nights). Data are graphed as averages±standard deviations.
FIG. 3.
Effects of kudzu root extract on number of sleep episodes (A: kudzu, n=76 nights; placebo, n=71 nights), time asleep per episode (B: kudzu, n=74 nights; placebo, n=68 nights), and the number of immobile minutes (C: kudzu, n=75 nights; placebo, n=71 nights). Data are graphed as averages±standard deviations.
Alcohol craving and use
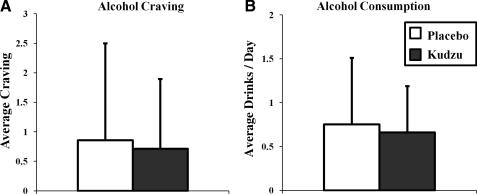
In order to assess alcohol use and craving, participants were asked to use the wrist actigraphy device to rate their subjective craving approximately once every 3 hours±20 minutes on a scale of 1 “not at all” to 10 “extremely.” In addition, they used this same wrist actigraphy device to report each alcoholic drink they consumed as they went about their daily lives. In these moderate drinkers, neither craving (Fig. 4A; F(1,690)=1.832, p=0.176) nor the number of drinks consumed per day for each participant (Fig. 4B; F(1,18)=0.103, p=0.752) were affected by 9 days of kudzu root treatment.
FIG. 4.
Effects of kudzu root extract on average craving (A: kudzu, n=368 assessments; placebo, n=324 assessments) and average number of drinks consumed per day (B: kudzu, n=10 participants; placebo, n=10 participants). Data are graphed as averages±standard deviations.
Discussion
Taken together, the data in this study demonstrate that in moderate drinkers, kudzu root extract treatment has no effect on any of the measured parameters of sleep quality or quantity. Sleep latency, sleep efficiency, and waking after sleep onset were all similar during placebo and kudzu root extract treatment periods (Fig. 1). Kudzu root extract did not increase nighttime wakefulness or movement, measured by the number of waking episodes per night, the time awake per episode, and the time spent mobile (Fig. 2). Finally, kudzu root extract did not affect sleep quantity as measured by the number of sleep episodes, the time asleep per episode, and the time spent immobile (Fig. 3).
It has previously been shown that kudzu root extract treatment decreases alcohol consumption in heavy drinkers9 whereas craving was unaffected. The current study was designed to assess any effect kudzu root extract treatment has on sleep in a population free of the sleep disturbances associated with alcohol withdrawal. In the moderate drinkers examined here, who were free to go about their daily lives and who were not attempting to decrease their alcohol consumption, there was no effect of kudzu root extract on alcohol consumption or craving (Fig. 4). This is important to the current study because the aim was to assess any effects kudzu root extract may itself have on sleep. If these participants had actually reduced their alcohol consumption, there might have been an impact on sleep, but it would have been difficult to separate the effect of kudzu root extract and the effect of withdrawal from alcohol.
A growing body of literature suggests that kudzu root extract may be an effective treatment to aid in drinking cessation.1–6,9 Withdrawal from alcohol triggers disturbances to sleep, and these sleep disturbances are associated with an increase in the likelihood of a relapse to drinking behavior.11,13,17,18 Most medications for treating alcohol dependence also have an effect on sleep.12–15 Thus, it is important to determine any effect of a candidate medication for alcohol dependence, such as kudzu root extract, on sleep quality and quantity. If a precipitating factor of relapse is the disturbed sleep associated with alcohol withdrawal and a medication used to treat alcohol withdrawal further disrupts sleep, then patients could experience a “double whammy” effect from the medication, further challenging their attempt to quit drinking. Thus, it is important that any novel medication by itself does not disturb sleep. This is the first study to address the effects of kudzu root extract treatment on the important variables of sleep quality and quantity.
With this said, there were a number of limitations to this study. It is not known whether kudzu root extract has an effect on sleep stages, because this study did not record night-time polysomnography or assess sleep architecture. The wrist actigraphy device was used instead for a number of reasons. First, this method allowed us to assess sleep continuously for the entire 9-day treatment periods. Second, one goal of this study was to measure behavior and craving while participants went about their daily lives, and use of the wrist actigraphy device allowed this assessment to take place in the participants' home environments. Third, this study already required participants to make several visits to the lab for measurements of alcohol metabolism and intoxication after alcohol administration. Therefore, the added requirement of spending several nights sleeping at the laboratory would have affected the normal behavior of participants, and added an unreasonable burden. A second limitation of this study is that these results rely on self-reported bedtimes and wake times and thus are subject to the limitations of any study for which behavior is not directly observed. However, because one aim of the study was to examine behavior of participants in their typical environments, this limitation was necessary.
Conclusions
Taken together, these data suggest that the administration of kudzu root extract does not disturb sleep/wake cycles of moderate drinkers and as such, its utility as an adjunct treatment for alcohol dependence remains free of any hidden side-effects on sleep quality. These results provide further evidence that kudzu root extract is likely to be safe for treatment for alcohol dependence.
Acknowledgments
This research was supported by NIDA grants K05 DA000343, T32 DA15036, and NIAAA grant AA10536. We thank Ronna Shostak, Carol Buchanan, and Barbara Beake for administrative support in conducting these studies.
Disclosure Statement
McLean Hospital has just negotiated a licensing agreement for kudzu root extract with Natural Pharmacia International, Inc., Burlington, MA. No competing financial interest exists for Bethany K. Bracken, David M. Penetar, R. Ross Maclean, or Scott E. Lukas.
References
- 1.Overstreet DH. Lee YW. Rezvani AH, et al. Suppression of alcohol intake after administration of the Chinese herbal medicine, NPI-028, and its derivatives. Alcohol Clin Exp Res. 1996;20:221–227. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 2.Overstreet D. Yee Y-W. Chen Y, et al. The Chinese herbal medicine NPI-028 suppresses alcohol intake in alcohol-preferring rats and monkeys without inducing taste aversion. J Perfusion. 1998;11:381–389. [Google Scholar]
- 3.Keung WM. Lazo O. Kunze L, et al. Daidzin suppresses ethanol consumption by Syrian golden hamsters without blocking acetaldehyde metabolism. Proc Natl Acad Sci U S A. 1995;92:8990–8993. doi: 10.1073/pnas.92.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keung WM. Vallee BL. Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc Natl Acad Sci U S A. 1993;90:10008–10012. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyman GM. Keung WM. Vallee BL. Daidzin decreases ethanol consumption in rats. Alcohol Clin Exp Res. 1996;20:1083–1087. doi: 10.1111/j.1530-0277.1996.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin RC. Guthrie S. Xie CY, et al. Isoflavonoid compounds extracted from Pueraria lobata suppress alcohol preference in a pharmacogenetic rat model of alcoholism. Alcohol Clin Exp Res. 1996;20:659–663. doi: 10.1111/j.1530-0277.1996.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KD. Brown NM. Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 suppl):1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 8.Lukas S. Human studies of kudzu as a treatment for alcohol abuse. In: Keung W, editor. Pueraria: The Genus Pueraria. New York: Taylor and Francis; 2002. pp. 159–179. [Google Scholar]
- 9.Lukas SE. Penetar D. Berko J, et al. An extract of the Chinese herbal root kudzu reduces alcohol drinking by heavy drinkers in a naturalistic setting. Alcohol Clin Exp Res. 2005;29:756–762. doi: 10.1097/01.alc.0000163499.64347.92. [DOI] [PubMed] [Google Scholar]
- 10.Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- 11.Feige B. Scaal S. Hornyak M, et al. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31:19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Gann H. Feige B. Cloot O, et al. Polysomnography during withdrawal with clomethiazole or placebo in alcohol dependent patients: A double-blind and randomized study. Pharmacopsychiatry. 2004;37:228–235. doi: 10.1055/s-2004-832597. [DOI] [PubMed] [Google Scholar]
- 13.Malcolm R. Myrick LH. Veatch LM, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: A randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3:24–32. [PubMed] [Google Scholar]
- 14.Staner L. Boeijinga P. Danel T, et al. Effects of acamprosate on sleep during alcohol withdrawal: A double-blind placebo-controlled polysomnographic study in alcohol-dependent subjects. Alcohol Clin Exp Res. 2006;30:1492–1499. doi: 10.1111/j.1530-0277.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 15.Schneider U. Wohlfahrt K. Schulze-Bonhage A, et al. Lack of psychotomimetic or impairing effects on psychomotor performance of acamprosate. Pharmacopsychiatry. 1998;31:110–113. doi: 10.1055/s-2007-979309. [DOI] [PubMed] [Google Scholar]
- 16.Brower KJ. Myra Kim H. Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32:1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brower KJ. Aldrich MS. Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- 18.Brower KJ. Aldrich MS. Robinson EA, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First M. Spitzer R. Gibbon M, et al. New York: New York State Psychiatric Institute, Biometrics Research; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) [Google Scholar]
- 20.Del Boca FK. Kranzler HR. Brown J, et al. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 21.Mini Mitter Company, Inc. Sunriver, OR: Mini Mitter Company, Inc.; 2000. Instruction Manual: Software Version 3.0 and Earlier. [Google Scholar]