Figure 3.
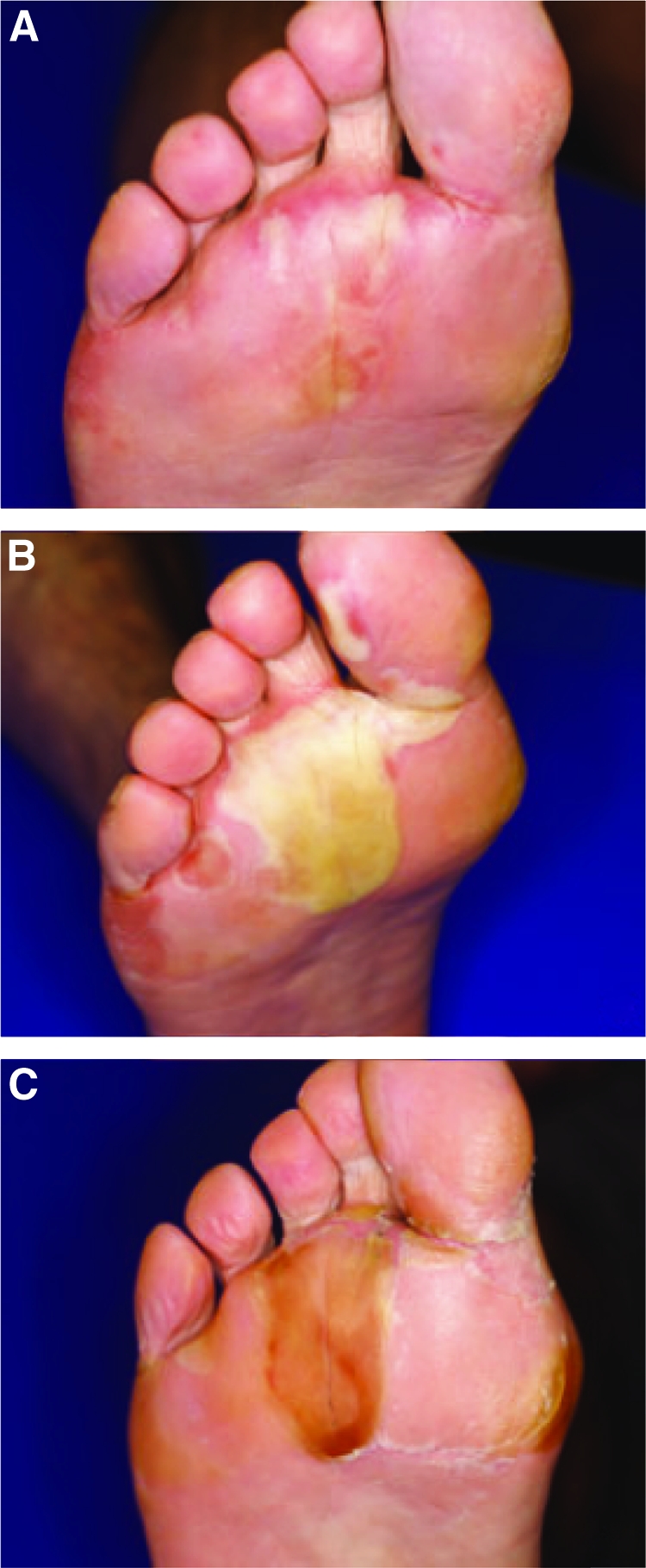
Time course of hand–foot skin reaction (HFSR) associated with sorafenib. (A): Early HFSR after 1 week of 400 mg sorafenib twice daily. (B): Progression of HFSR despite reduction to 400 mg daily and topical mometasone. (C): Sorafenib dosing interrupted, improvement in HFSR within 4 days. After 1 week of interruption, sorafenib was restarted at 400 mg daily without further complications. Reprinted from Degen A, Alter M, Schenck F et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges 2010;8:652–661, with permission.
