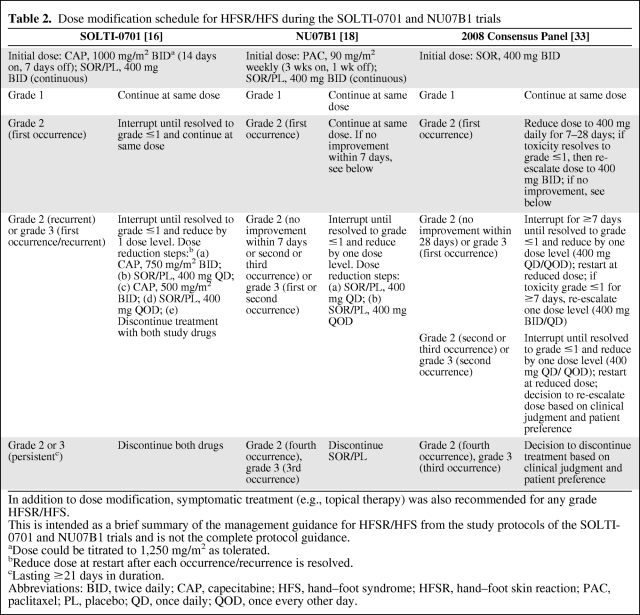
Table 2.
Dose modification schedule for HFSR/HFS during the SOLTI-0701 and NU07B1 trials
In addition to dose modification, symptomatic treatment (e.g., topical therapy) was also recommended for any grade HFSR/HFS.
This is intended as a brief summary of the management guidance for HFSR/HFS from the study protocols of the SOLTI-0701 and NU07B1 trials and is not the complete protocol guidance.
aDose could be titrated to 1,250 mg/m2 as tolerated.
bReduce dose at restart after each occurrence/recurrence is resolved.
cLasting ≥21 days in duration.
Abbreviations: BID, twice daily; CAP, capecitabine; HFS, hand–foot syndrome; HFSR, hand–foot skin reaction; PAC, paclitaxel; PL, placebo; QD, once daily; QOD, once every other day.