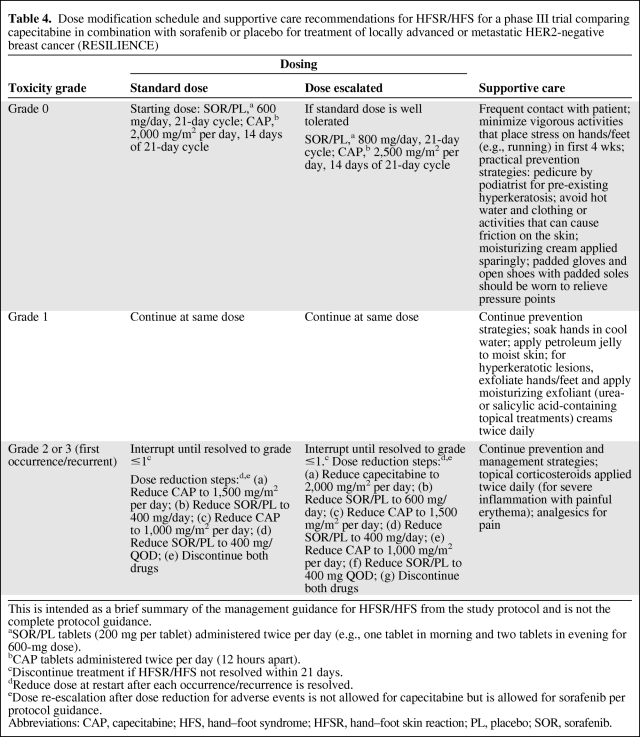
Table 4.
Dose modification schedule and supportive care recommendations for HFSR/HFS for a phase III trial comparing capecitabine in combination with sorafenib or placebo for treatment of locally advanced or metastatic HER2-negative breast cancer (RESILIENCE)
This is intended as a brief summary of the management guidance for HFSR/HFS from the study protocol and is not the complete protocol guidance.
aSOR/PL tablets (200 mg per tablet) administered twice per day (e.g., one tablet in morning and two tablets in evening for 600-mg dose).
bCAP tablets administered twice per day (12 hours apart).
cDiscontinue treatment if HFSR/HFS not resolved within 21 days.
dReduce dose at restart after each occurrence/recurrence is resolved.
eDose re-escalation after dose reduction for adverse events is not allowed for capecitabine but is allowed for sorafenib per protocol guidance.
Abbreviations: CAP, capecitabine; HFS, hand–foot syndrome; HFSR, hand–foot skin reaction; PL, placebo; SOR, sorafenib.