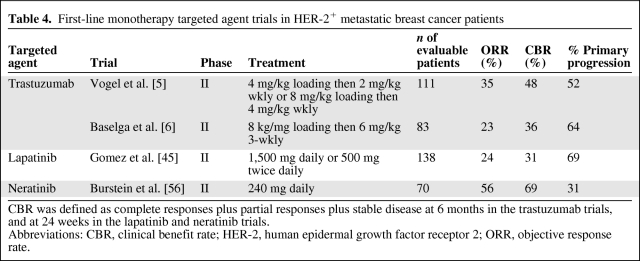
Table 4.
First-line monotherapy targeted agent trials in HER-2+ metastatic breast cancer patients
CBR was defined as complete responses plus partial responses plus stable disease at 6 months in the trastuzumab trials, and at 24 weeks in the lapatinib and neratinib trials.
Abbreviations: CBR, clinical benefit rate; HER-2, human epidermal growth factor receptor 2; ORR, objective response rate.