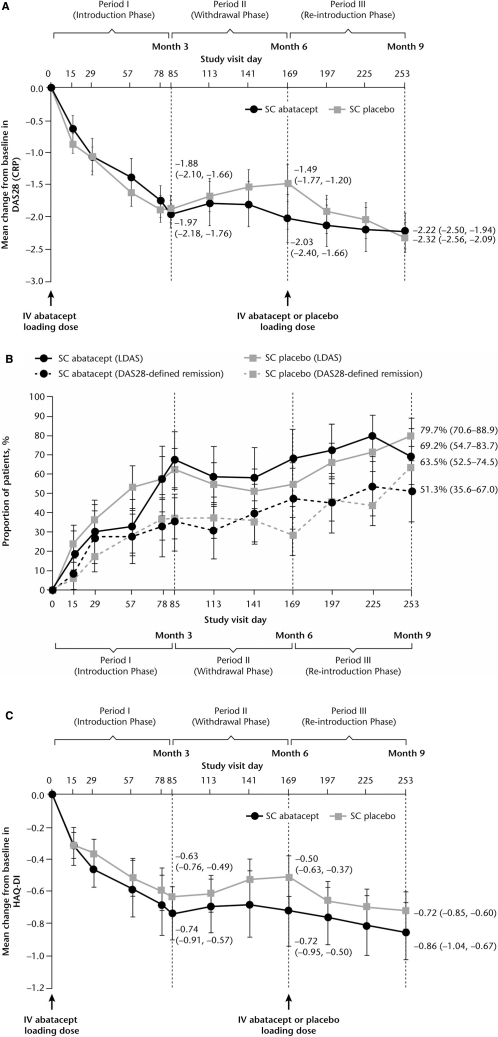
Figure 3.
Clinical efficacy. (A) Mean change in DAS28 (CRP) over time by period II (Withdrawal phase) treatment group. (B) Proportion of patients achieving LDAS and DAS28-defined remission by period II (Withdrawal phase) treatment group. (C) Mean change in HAQ-DI score over time by period II (Withdrawal phase) treatment group. Data are as-observed for all patients who received ≥1 dose of study drug during period II. Error bars represent 95% CI. CRP, C reactive protein; DAS28, Disease Activity Score 28; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDAS, low disease activity state; SC, subcutaneous.