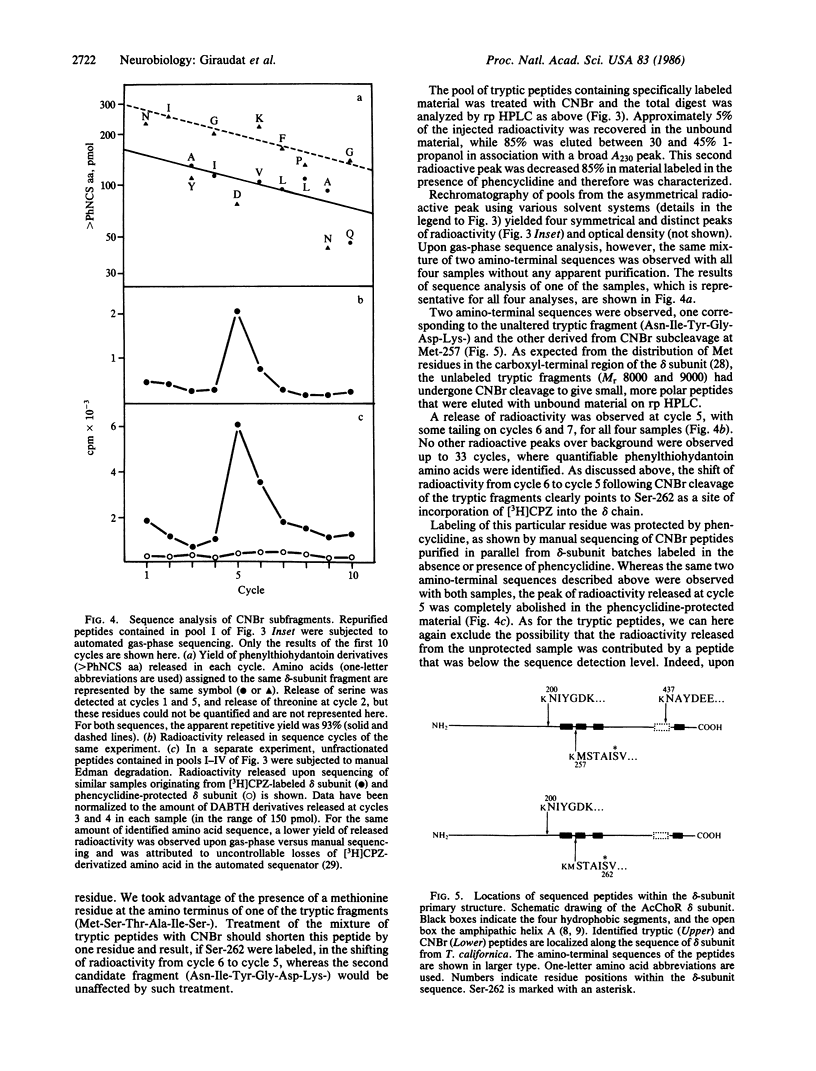
Abstract
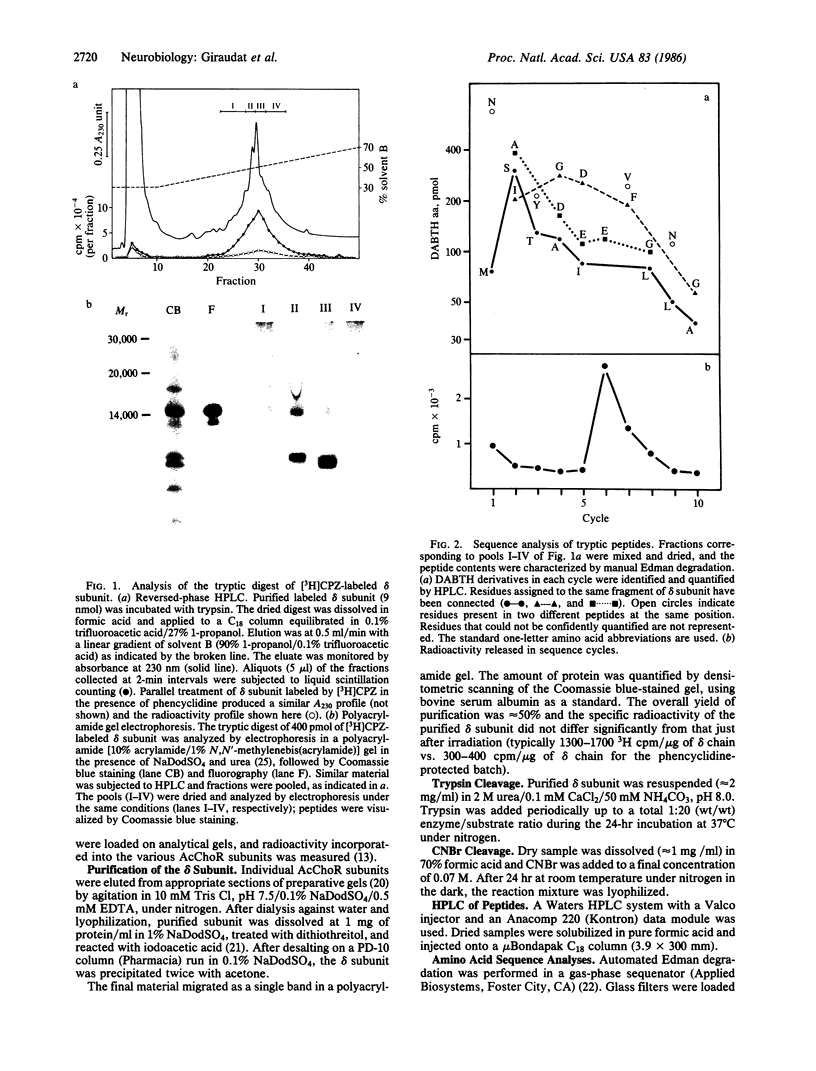
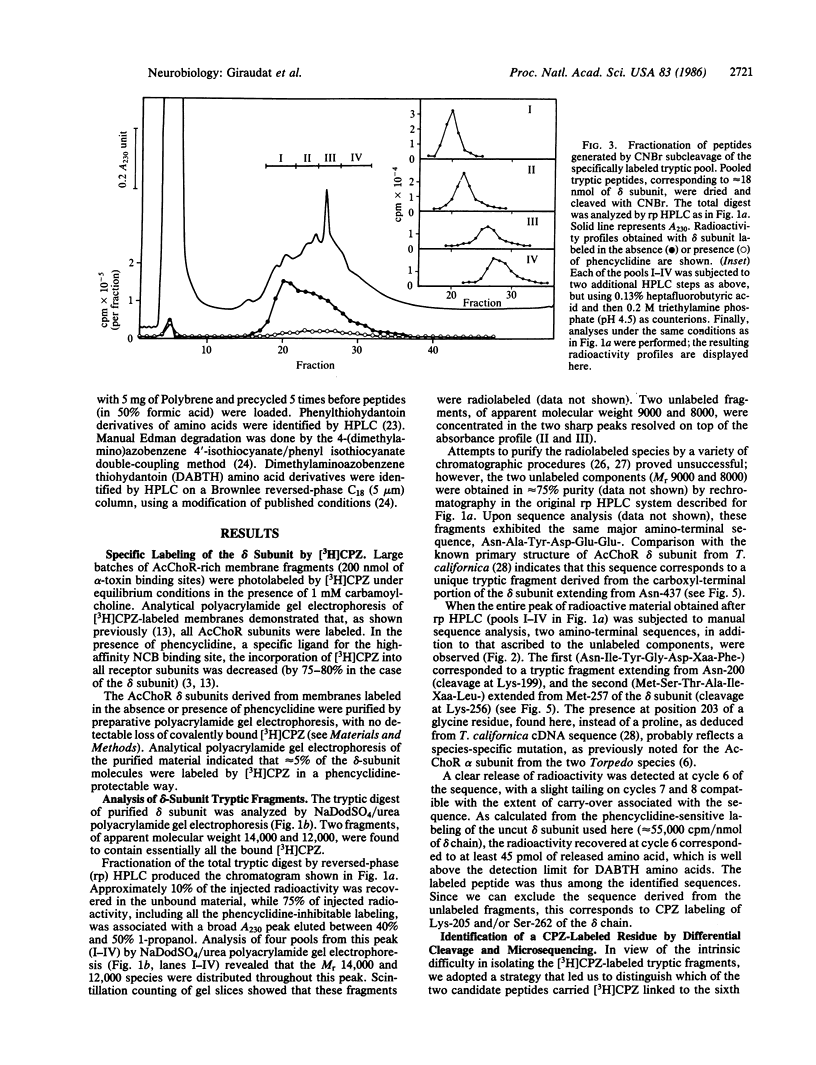
The membrane-bound acetylcholine receptor from Torpedo marmorata was photolabeled by the noncompetitive channel blocker [3H]chlorpromazine under equilibrium conditions in the presence of agonist. Incorporation of radioactivity into all subunits occurred and was reduced by addition of phencyclidine, a specific ligand for the high-affinity site for noncompetitive blockers. The delta subunit was purified and digested with trypsin, and the resulting fragments were fractionated by reversed-phase HPLC. The labeled peptide could not be purified to homogeneity because of its marked hydrophobic character, but a combination of differential CNBr subcleavage and cosequencing of partially purified fragments enabled us to identify Ser-262 as being labeled by [3H]chlorpromazine. The labeling of this particular residue was prevented by phencyclidine and thus took place at the level of, or in proximity to, the high-affinity site for noncompetitive blockers. Ser-262 is located in a hydrophobic and potentially transmembrane segment termed MII.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Desensitization of membrane-bound Torpedo acetylcholine receptor by amine noncompetitive antagonists and aliphatic alcohols: studies of [3H]acetylcholine binding and 22Na+ ion fluxes. Biochemistry. 1984 Aug 28;23(18):4023–4033. doi: 10.1021/bi00313a003. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Manual micro-sequence analysis of polypeptides using dimethylaminoazobenzene isothiocyanate. Methods Enzymol. 1983;91:455–466. doi: 10.1016/s0076-6879(83)91043-1. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Changeux J. P., Paroutaud P., Strosberg A. D. The amino-terminal sequence of the 40,000 molecular weight subunit of the acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 1979 Aug 1;104(1):99–105. doi: 10.1016/0014-5793(79)81092-3. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünhagen H. H., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. IV. Quinacrine: a fluorescent probe for the conformational transitions of the cholinergic receptor protein in its membrane-bound state. J Mol Biol. 1976 Sep 25;106(3):497–516. doi: 10.1016/0022-2836(76)90249-7. [DOI] [PubMed] [Google Scholar]
- Guy H. R. A structural model of the acetylcholine receptor channel based on partition energy and helix packing calculations. Biophys J. 1984 Jan;45(1):249–261. doi: 10.1016/S0006-3495(84)84152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring R., Kloog Y., Kalir A., Sokolovsky M. Species differences determine azido phencyclidine labeling pattern in desensitized nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 1983 Jun 15;113(2):723–729. doi: 10.1016/0006-291x(83)91786-2. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Fast kinetic studies on the allosteric interactions between acetylcholine receptor and local anesthetic binding sites. Eur J Biochem. 1979 Feb 15;94(1):281–296. doi: 10.1111/j.1432-1033.1979.tb12894.x. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Time-resolved photolabeling by the noncompetitive blocker chlorpromazine of the acetylcholine receptor in its transiently open and closed ion channel conformations. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1897–1901. doi: 10.1073/pnas.81.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Kaldany R. R., Karlin A. Reaction of quinacrine mustard with the acetylcholine receptor from Torpedo californica. J Biol Chem. 1983 May 25;258(10):6232–6242. [PubMed] [Google Scholar]
- Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. Identification of the alpha subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J Biol Chem. 1984 Oct 10;259(19):11662–11665. [PubMed] [Google Scholar]
- Knecht R., Seemüller U., Liersch M., Fritz H., Braun D. G., Chang J. Y. Sequence determination of eglin C using combined microtechniques of amino acid analysis, peptide isolation, and automatic Edman degradation. Anal Biochem. 1983 Apr 1;130(1):65–71. doi: 10.1016/0003-2697(83)90650-4. [DOI] [PubMed] [Google Scholar]
- Krodel E. K., Beckman R. A., Cohen J. B. Identification of a local anesthetic binding site in nicotinic post-synaptic membranes isolated from Torpedo marmorata electric tissue. Mol Pharmacol. 1979 Mar;15(2):294–312. [PubMed] [Google Scholar]
- Kubo T., Noda M., Takai T., Tanabe T., Kayano T., Shimizu S., Tanaka K., Takahashi H., Hirose T., Inayama S. Primary structure of delta subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur J Biochem. 1985 May 15;149(1):5–13. doi: 10.1111/j.1432-1033.1985.tb08885.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muhn P., Hucho F. Covalent labeling of the acetylcholine receptor from Torpedo electric tissue with the channel blocker [3H]triphenylmethylphosphonium by ultraviolet irradiation. Biochemistry. 1983 Jan 18;22(2):421–425. doi: 10.1021/bi00271a028. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Oswald R. E., Changeux J. P. Selective labeling of the delta subunit of the acetylcholine receptor by a covalent local anesthetic. Biochemistry. 1981 Dec 8;20(25):7166–7174. doi: 10.1021/bi00528a018. [DOI] [PubMed] [Google Scholar]
- Oswald R., Changeux J. P. Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3925–3929. doi: 10.1073/pnas.78.6.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M. W., Lukas T. J., Cohen S., Staros J. V. Identification of residues in the nucleotide binding site of the epidermal growth factor receptor/kinase. J Biol Chem. 1985 May 10;260(9):5205–5208. [PubMed] [Google Scholar]
- Saitoh T., Oswald R., Wennogle L. P., Changeux J. P. Conditions for the selective labelling of the 66 000 dalton chain of the acetylcholine receptor by the covalent non-competitive blocker 5-azido-[3H]trimethisoquin. FEBS Lett. 1980 Jul 11;116(1):30–36. doi: 10.1016/0014-5793(80)80522-9. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Gerber G. E., Nihei K., Khorana H. G. Amino acid sequence of the membranous segment of rabbit liver cytochrome b5. Methodology for separation of hydrophobic peptides. J Biol Chem. 1980 Feb 25;255(4):1536–1541. [PubMed] [Google Scholar]
- Tarr G. E., Crabb J. W. Reverse-phase high-performance liquid chromatography of hydrophobic proteins and fragments thereof. Anal Biochem. 1983 May;131(1):99–107. doi: 10.1016/0003-2697(83)90140-9. [DOI] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J Biol Chem. 1977 Nov 10;252(21):7648–7656. [PubMed] [Google Scholar]