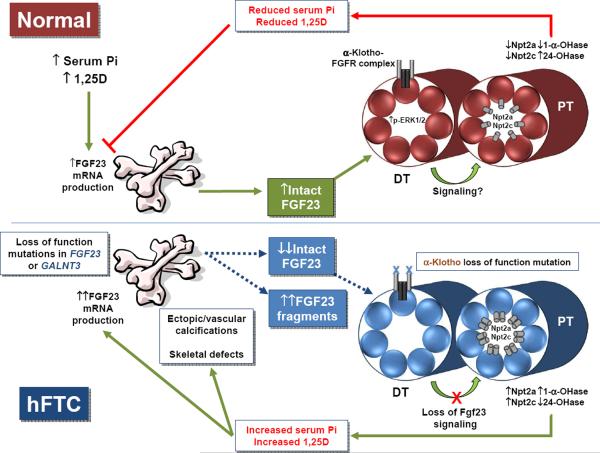
Figure 1.
(top panel) Under normal physiological conditions, increased serum phosphate and 1,25D stimulate FGF23 production in bone, increasing bioactive, intact serum FGF23. FGF23 circulates to the kidney where it binds to a receptor complex comprised of αKlotho and an FGFR in the distal tubule (DT), which then through an unknown mechanism causes a decrease in expression of the sodium phosphate co-transporters, Npt2a and Npt2c, in the proximal tubule (PT) apical membrane. Simultaneously, FGF23 increases expression of the vitamin D catabolic enzyme, 24-hydroxylase, and decreases 1-α-hydroxylase expression. Together, the decrease in Npt2a, Npt2c, and 1,25D leads to a decrease in serum phosphate, which in a negative feedback loop, decreases FGF23 production in the bone. (lower panel) In hFTC, loss of function mutations in either FGF23 or GALNT3 result in decreased intact serum FGF23, and an increase in inactive C-terminal FGF23 fragments. The inactive fragments likely do not signal in the kidney. The αKlotho loss of function mutation leads to a reduction in the activity of the FGF23 receptor signaling complex in the distal tubule. All of the TC mutations likely lead to a release of suppression of Npt2a and Npt2c, a decrease in 24-hydroxylase, and an increase in 1-α-hydroxylase expression, ultimately increasing serum phosphate concentrations, resulting in ectopic and vascular calcifications, and a further stimulus of FGF23 in bone. However, in contrast to FGF23 and GALNT3 mutations, the αKlotho loss of function mutation leads to an increase in serum intact FGF23, as FGF23 protein processing in bone is not directly affected by this mutation.