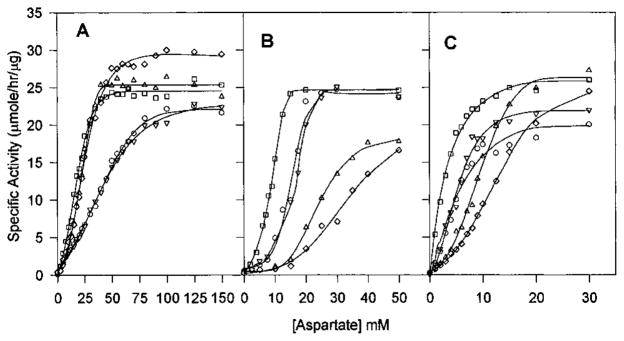
Figure 4.
Aspartate saturation kinetics in the presence of nucleotide effectors for native Sm (A), native Ec (B), and chimera SM:rS5′ec (C) ATCase holoenzyme. The aspartate saturation of ATCase activity was determined in the presence of (□) either 4 mM (for the native Sm enzyme) or 2 mM ATP (for the native Ec enzyme and the chimeric enzyme, respectively), (△) 2 mM CTP, (▽) 2 mM UTP, (◇) 2 mM CTP plus 2 mM UTP, and (○) without any nucleotide effector. Standard assay conditions were used, and each curve is the average of at least three independent assays.