Figure 5.
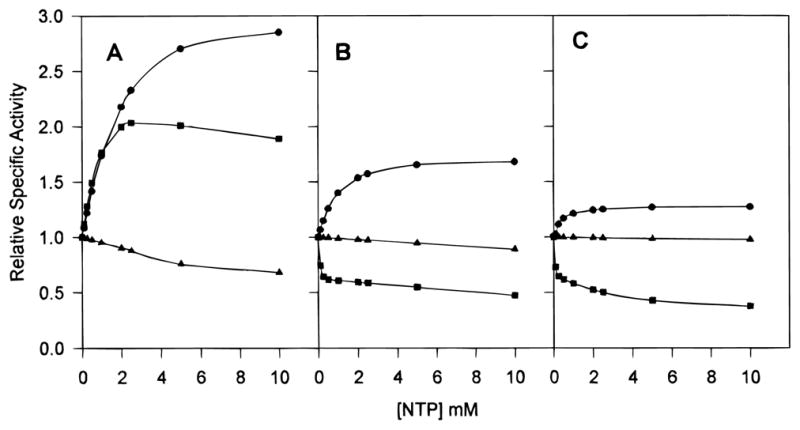
Comparative nucleotide saturation kinetics of native Sm (A), native Ec (B), and chimera SM:rS5′ec (C) ATCase holoenzyme. The saturation effects of ATP (●), CTP (■), and UTP (▲) on the activity of ATCase were determined under assay conditions employing saturating carbamoyl phosphate concentrations and aspartate concentrations at the [Asp]0.5 of each enzyme (4.8 mM carbamoyl phosphate for all enzymes; 39.2, 16.6, and 4.7 mM aspartate for native Sm, Ec, and chimera SM:rS5′ec enzymes, respectively). The specific activity was measured at each concentration of nucleotide (NTP) and plotted relative to the activity with no effector present.
