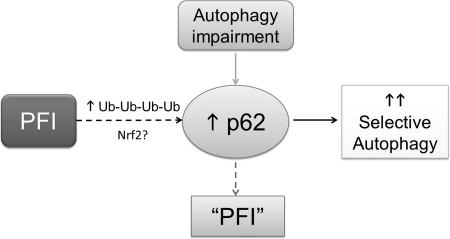
Fig. 4.
An illustration of a working hypothesis on the interplay between proteasome-mediated proteolysis and the selective autophagy. On the one hand, PFI accumulates ubiquitin conjugates which, through interaction, retain p62 proteins in the cell. PFI may also activate the nuclear factor erythroid-derived 2-related factor-1 (Nrf2)-mediated transcriptional program to activate p62 synthesis. The binding of p62 to ubiquitin conjugates in protein aggregates may recruit autophagosomes to and remove the aggregates, thereby increasing selective autophagy. On the other hand, chronic autophagy inhibition/impairment can accumulate p62, which is a substrate of autophagy. The accumulated p62 can bind to ubiquitinated proteins formed during normal ubiquitination and hinder them from being delivered to the proteasome for degradation; therefore, autophagy impairment may indirectly induce a PFI-like phenotype, although proteasome assembly and activities may not be decreased.