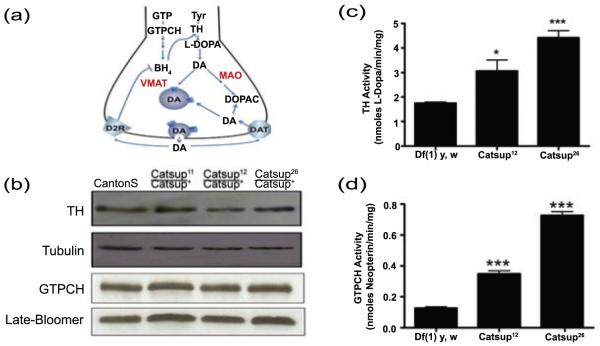
Figure 1.
Catsup mutations dominantly elevate TH and GTPCH activity through a post-translational mechanism. (a) Key components of DA synthesis, transport and metabolism. GTP is converted via three enzymatic reactions to the cofactor BH4. GTPCH catalyzes the rate-limiting step. TH converts tyrosine (tyr) to L-DOPA, in a BH4-dependent reaction. Aromatic amino acid decarboxylase subsequently converts L-DOPA to DA. DA is then transported into synaptic vesicles via VMAT or is converted by a two-step pathway into DOPAC by Monoamine Oxidase (MAO) and aldehyde dehydrogenase. After synaptic release, residual extracellular DA may be transported by into the pre-synaptic terminus by Dopamine Transporter (DAT) or may interact with the D2 autoreceptor (D2R) to trigger a G-protein coupled signaling pathway that leads to dephosphorylation of TH, which down-regulates its activity. (b) Immunoblot analysis of 3-5 day old flies was performed using antibodies against dTH, dGTPCH and loading controls, ß-tubulin and late-bloomer. Levels of TH and GTPCH protein are indistinguishable in the wild type (Canton S) and heterozygous Catsup mutant head extracts. (c, d) Extracts of adult male and female heads (in equal numbers), collected 24-48 hrs post-eclosion, were assayed for TH and GTPCH activity. Activities are expressed as nmoles of product per min. per mg protein. TH and GTPCH activities are elevated in head extracts of Catsup26/ Catsup+ and Catsup12/Catsup+ mutants relative to the wild type control (*, p< 0.05, **, p<0.01, *** p<0.001, n=5).