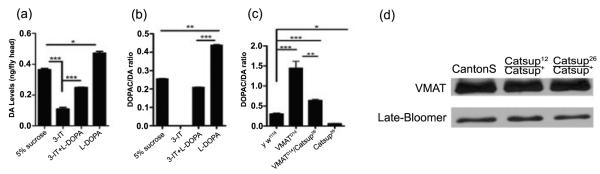
Figure 5.
Pharmacological and genetic manipulation of cytosolic dopamine pools. (a, b) Pharmacological modulation of DA levels in y w1118 flies. Adults, 48 h post-eclosion, were fed 5% sucrose, 30 mM 3-iodo-tyrosine (3-IT), 30 mM 3-IT + 1mM L-DOPA, or 1mM L-DOPA for 12 hrs. (a) DA levels were significantly depleted in the presence of 3-IT, and significantly elevated when fed L-DOPA. Co-feeding 3-IT and L-DOPA resulted in near normal DA pools. (b) DOPAC:DA ratios respond to pharmacological alteration of DA pools. The TH inhibitor, 3-IT, reduced the DOPAC:DA ratio to a negligible level, while flies treated with L-DOPA had significantly higher DOPAC:DA ratios relative to controls. These results indicate that removal of DA from the cytosol is not solely dependent on cytosolic DA levels. (c) Double mutant analysis of DOPAC:DA ratios in VMATD14/Catsup26 reveals a functional interaction. VMATD1 /VMAT+ and Catsup26/Catsup+ mutants display strongly elevated and reduced DOPAC:DA ratios, respectively, in comparison to the y w1118 flies. The transheterozygous combination of Catsup26 and VMATD14 mutations partially rescues the DOPAC:DA ratio relative to VMATD1 /VMAT+ flies. Statistical analysis was performed using one-way ANOVA. Error bars represent mean SEM. (*, p<0.05, **, p<0.01, ***, p<0.001, n=5). (d) Mutations in Catsup do not alter VMAT protein levels. Immunoblot analysis was performed using antibodies against dVMAT and loading control, Late-bloomer.