Abstract
Intermittent pneumatic leg compressions (IPC) have proven to be an effective noninvasive approach for treatment of patients with claudication, but the mechanisms underlying the clinical benefits remain elusive. In the present study, a rodent model of claudication produced by bilateral ligation of the femoral artery was used to investigate the acute impact of a single session of IPC (150 min) on hemodynamics, skeletal muscle (tibialis anterior), and isolated collateral artery (perforating artery) expression of a subset of genes associated with inflammation and vascular remodeling. In addition, the effect of compression frequency (15 vs. 3 compressions/min) on the expression of these factors was studied. In ligated animals, IPC evoked an increase of monocyte chemoattractant protein-1 (MCP-1) and cytokine-induced neutrophil chemoattractant 1 (CXCL1) mRNA (P < 0.01) and immunostaining (P < 0.05), as well as a minor increase in VEGF immunostaining in the muscle endomysium 150 min postintervention. Further, collateral arteries from these animals showed an increased expression of MCP-1 (approximately twofold, P = 0.02). These effects were most evident in the group exposed to the high-frequency protocol (15 compressions/min). In contrast, IPC in sham-operated control animals evoked a modest initial upregulation of VEGF (P = 0.01), MCP-1 (P = 0.02), and CXCL1 (P = 0.03) mRNA in the muscle without concomitant changes in protein levels. No changes in gene expression were observed in arteries isolated from sham animals. In conclusion, IPC acutely up-regulates the expression of important factors involved in vascular remodeling in the compressed muscle and collateral arteries in a model of hindlimb ischemia. These effects appear to be dependent on the compression frequency, such that a high compression frequency (15 compressions/min) evokes more consistent and robust effects compared with the frequency commonly employed clinically to treat patients with claudication (3 compressions/min).
Keywords: intermittent pneumatic compression, claudication
application of intermittent pneumatic leg compressions (IPC) has recently emerged as a promising alternative therapy for patients with intermittent claudication. Because IPC is home-based, this treatment strategy overcomes the major limitations associated with the surgical and noninvasive therapeutic options available for these patients, including low accessibility and unsatisfactory cost-effectiveness (6, 17). Clinical trials in stable claudicants indicate that 3 mo of daily IPC application can increase maximal walking distance by ∼110% (5, 9, 27), an improvement that is comparable to that obtained by supervised exercise training (17, 32). Despite the growing clinical acceptance, the fundamental mechanisms by which IPC therapy promotes clinical benefits remain unclear. In addition, there is a paucity of data describing the optimum stimulation characteristics (e.g., compression timing and pressure), for claudicants.
One prevailing hypothesis is that increases in exercise tolerance associated with IPC treatment are mostly related to the enlargement and/or expansion of the arterial collateral network of the ischemic limb evoked by this therapy (6, 9). This hypothesis is supported by results of experiments by van Bemmelen et al. (39) in a preclinical model of limb ischemia. These authors exposed rabbits to 10 wk of daily IPC (1 h/day) and detected a marked increase in the number of collaterals perfusing the calf, as revealed by angiography. The exact mechanisms by which IPC promotes arteriogenesis are unclear, but it has been proposed that repeated increases in leg blood flow and shear-stress during this intervention could play a role (8, 40). Collateral remodeling in arterial obstructive diseases is known to involve a complex, orchestrated series of events initiated by shear-stress and circumferential wall tension (11). One key event in this process is the initial local up-regulation of genes encoding chemokines/cytokines and activation of endothelial cells by increased shear-stress (4, 11). Conceivably, IPC application-induced changes in vascular hemodynamic forces can initiate the cascade of events that are typically associated with arteriogenesis. To date, however, no study has investigated the molecular responses to IPC therapy in collateral arteries.
Accumulating evidence also indicates that besides affecting the collateral network, IPC treatment can impact the compressed tissue. Data from Tan et al. (34) first demonstrated that a single session of IPC in rats increased endothelial nitric oxide synthase mRNA and protein expression in the compressed tibialis anterior (TA) muscle (34). In line with this finding, we recently reported that a single session of IPC evokes a frequency-dependent increase in vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein-1/(C-C motif) ligand 2 (MCP-1/Ccl2) mRNA expression in the TA in rats (28). However, these studies were conducted in healthy animals, and it is thus unclear whether the same pattern of responses holds true in a model of peripheral arterial insufficiency, in which a vast array of changes in gene expression occur in the ischemic muscle (26)/(C-C motif) ligand 2.
The purpose of the present study was to use an established model of peripheral arterial insufficiency (43) to accomplish three major aims. First, we used collateral arteries isolated from bilateral occluded rats to test the hypothesis that a single session of IPC application would alter the mRNA expression of a subset of chemokines that have shown to be among the most robustly up-regulated factors early in the process of arteriogenesis (4). We also evaluated the time course and magnitude of IPC-induced changes in mRNA and protein expression of VEGF, MCP-1, and cytokine-induced neutrophil chemoattractant 1 (CXCL1/CINC-1) in the TA muscle. The second aim was to test the hypothesis that in limbs of chronic bilateral occluded rats, a higher compression frequency would evoke more robust changes in the expression of the aforementioned factors than 3 compressions/min, which is the frequency currently employed in commercially available devices (5, 9, 17, 27). This hypothesis was based on previous results in healthy animals (28), where the higher frequency of compressions had greater effects on the expression of these genes. Third, to gain insights into the possible role of hemodynamic signals in promoting changes in gene expression in the muscle and in collateral arteries, we performed experiments to characterize the hemodynamic changes evoked by IPC application.
METHODS
Animals.
Male Sprague-Dawley rats (300–350 g body wt) were used in the present investigation. Animals were housed in pairs in temperature- (24°C) and light (12:12-h light-dark cycle)-controlled rooms. Rat chow and water were available ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri.
Femoral artery ligation.
Bilateral ligation of the femoral artery was performed as described previously (26). Animals were anesthetized with a mixture of ketamine (100 mg/kg) and acepromazine (0.5 mg/kg), and their femoral arteries were exposed and isolated immediately distal to the inguinal ligament. A ligature (3–0 silk) was placed tightly around each femoral artery, ∼3 mm distal to the inguinal ligament. In the sham-operated animals, a similar surgical procedure was performed except for the ligation of the artery. The animals were allowed 1 wk of recovery before the experiments to minimize the confounding effect of the surgery on muscle and artery gene expression. In this model, collateral-dependent blood flow in sedentary animals reaches near peak values (∼90%) within 1 wk after ligation (26).
Experimental design.
We recently described a model in rats to study the acute effects of a single session of IPC applied to the calf (28). In this animal model, a small blood pressure cuff (UPC3.3, 3.3 × 12 cm; D. E. Hokanson, Bellevue, WA) is firmly wrapped around the left calf, and IPC is applied continuously for 150 min. This time duration is similar to that prescribed in the available clinical trials in stable claudicants (5, 9, 17). In healthy animals, this approach was reported to evoke leg blood flow responses analogous to that seen in humans under the same conditions (28). To determine the time course of changes in gene expression in the muscle, two subsets of experiments were performed. In the first subset (n = 24), the tissues were harvested immediately after the end of the 150 min IPC session (0 min), and in the second subset (n = 24), the samples were taken 150 min following the completion of the protocol. In each subset, animals were divided in two conditions: bilateral ligation of the femoral artery and sham-operated controls, with 12 or 13 animals in each group. Animals in each condition were randomly assigned to one of three compression protocols: 120 mmHg (2 s inflation/2 s deflation), 120 mmHg (4 s inflation/16 s deflation), and controls (no compression). In accordance with our previous report (28) and other reports (5), we studied gene/protein expression in the left TA muscle of the rat. In addition, the rat perforating artery, which supplies flow to the distal hamstring muscles, was utilized to evaluate the effects of IPC on the collateral network. This artery has been shown to be part of the collateral circuit in rats after ligation of the femoral artery (13, 26). In preliminary experiments, we did not observe changes in gene expression in the arteries harvested promptly after the end of the IPC bout, and accordingly, in the present experiment, we only studied the arteries of the subset of animals in which the tissues were harvested 150 min following completion of the IPC session. To characterize the acute blood flow responses to the application of compressions, an additional group of animals (n = 10) was studied.
Surgical preparation.
On the day of the experiment, the animals were anesthetized with a combination of ketamine (100 mg/kg) and acepromazine (0.5 mg/kg ip), and a catheter (polyethylene-50 tubing) was placed in the left carotid artery for blood pressure determination. The animals were prepared for IPC application, as previously described (28). Briefly, to stabilize the leg and prevent movement of the cuff during compressions, umbilical tape was passed in the ankle region between the bone and the Achilles tendon and tied tightly to a metal rod that was connected to a force transducer. The cuff was also secured to the leg with umbilical tape. During the surgical procedures, as well as during the IPC session, an adequate level of anesthesia was verified by the lack of response to foot pinch, stable ventilation, and stable blood pressure. Additional doses were given throughout the intervention period if and when necessary. Core temperature was maintained at 37°C with an external heat source.
IPC device.
An automatic cuff inflator (E-20 rapid cuff inflator; D. E. Hokanson) was used for IPC application (28). With the cuff used in the present study (UPC3.3, 3.3 × 12 cm; D. E. Hokanson), the inflation/deflation time is <0.1 s. A computer-generated signal (PowerLab 4/S system; ADInstruments, Colorado Springs, CO) allowed for application of the compression rates specified above.
Tissue processing.
At the end of the intervention period, the compression cuff was removed, and the left TA muscle was harvested immediately or 150 min following the intervention. The muscle was promptly divided into three sections: one section (∼30 mg) was placed in RNA stabilization reagent (RNAlater; Ambion, Austin, TX) and kept at 4°C for up to a week until further processing for RT-PCR analysis; a second section (∼800 mg) was snap frozen in liquid nitrogen and stored at −80°C, and a third section was immersed in neutral-buffered 10% formalin and saved for immunohistochemistry analysis. Subsequently, the entire limb was disarticulated at the level of hip joint, rinsed in cold physiological saline solution and placed in a chamber containing cold (4°C) RNA stabilization reagent (RNAlater; Ambion). Under a dissecting microscope, a segment of the left perforating artery was dissected away from overlying muscle tissue, placed individually in ribonuclease-free microcentrifuge tubes, and frozen at −80°C for subsequent RT-PCR analysis.
RNA isolation and RT-PCR analysis.
Approximately 30 mg of the muscle tissue was homogenized in a lysing solution (Buffer RLT; Qiagen, Valencia, CA) containing 14.3 M β-mercaptoethanol (β-ME) using a tissue homogenizer (Fisher Scientific, Waltham, MA), as previously described (28). Isolated segments of the left perforating artery were initially minced under a lysing solution with small scissors followed by homogenization using a minipestle fitted in Pellet Pestle Motor (Kontes,Vineland, NJ). Total RNA was isolated using an RNeasy fibrous tissue mini (muscle samples) or micro (arteries) kits (Qiagen, Valencia, CA) and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA by reverse transcription primed by a mixture of random hexamer and oligo(dT) primers (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA). The reactions were incubated in a PCR Express Hybaid thermal cycler (Hybaid, Franklin, MA). Quantitative real-time PCR was performed using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers for each target were purchased from IDT (Coralville, IA). Sequences used are outlined in Table 1. A 25-μl reaction mixture containing 24 μl (muscle) or 22 μl (arteries) of Power SYBR Green PCR Master Mix (Applied Biosystems), and the appropriate concentrations of gene-specific primers plus 1 μl (muscle) or 3 μl (arteries) of cDNA template were loaded in each well of a 96-well plate (duplicate samples). PCR was performed with thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. A dissociation curve analysis was performed after each run to verify the identity of the PCR products. The comparative cycle threshold (Ct) method was utilized to calculate the changes in expression of each target mRNA (19).
Table 1.
Primers used in real-time PCR
| Gene | Forward Primers | Reverse Primers | Ref. |
|---|---|---|---|
| VEGF | 5′-TTCAAGCCGTCCTGTGTGC-3′ | 5′-TCCAGGGCTTCATCATTGC-3′ | (20) |
| MCP-1 | 5′-CTGTCTCAGCCAGATGCAGTTAA-3′ | 5′-AGCCGACTCATTGGGATCAT-3′ | (23) |
| CINC1 | 5′-CCAAAAGATGCTAAAGGGTGTCC-3′ | 5′-CAGAAGCCAGCGTTCACCA-3′ | (3) |
| IP-10 | 5′-CAGAAGCACCATGAACCCAAG-3′ | 5′-TCAACATGCGGACAGGATAGA C-3′ | (21) |
| MIP-1α | 5′-ATATGGAGCTGACACCCCGACT-3′ | 5′-CCAGCTCAGTGATGTATTCTTGGA-3′ | (21) |
| CXCL2 | 5′-GCAAGGCTAACTGACCTGGAA A-3′ | 5′-CAGGTACGATCCAGGCTTCCT-3′ | (37) |
| GAPDH | 5′-ACTCTACCCACGGCAAGTTC-3′ | 5-′TACTCAGCACCAGCATCACC-3′ | (28) |
Immunohistochemistry.
Sections of the TA muscle were kept under neutral-buffered 10% formalin for ≥24 h and then processed to paraffin embedment. Five-micrometer sections were cut with an automated microtome (Microm, Thermo Fischer Scientific, Bellefonte, PA), floated onto positively charged slides (Thermo Fischer Scientific), and deparaffinized. The slides were then steamed in citrate buffer at pH 6.0 (Dako target retrieval solution S1699; Dako, Carpenteria, CA) for 30 min to achieve antigen retrieval and subsequently cooled for 30 min. Next, the slides were stained manually with sequential Tris buffer, and water wash steps were performed after each protocol step. Sections were incubated with avidin biotin two-step blocking solution (Vector SP-2001; Vector Laboratories, Burlingame, CA) to inhibit background staining and in 3% hydrogen peroxide to inhibit endogenous peroxidase. Nonserum protein block (Dako X909; Dako) was applied to inhibit nonspecific protein binding. Immunostaining was performed using rabbit anti-rat VEGF (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-rat MCP-1 (1:100 dilution, PeproTech , Rocky Hill, NJ), or rabbit anti-rat CXCL1 (1:200 dilution; PeproTech) incubated with the tissue sections overnight at 4°C. Sections were examined using an Olympus BX60 photomicroscope (Olympus, Melville, NY) and photographed with Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI) at ×40 magnification. In each sample, Image Pro Plus software was used to quantify the positive area staining. An experienced investigator that was blinded to treatment group's identities performed the immunostaining analysis.
Hemodynamic measurements.
In an additional subset of animals (n = 10), experiments were performed to characterize the hemodynamic responses to a brief (6 min) exposure to IPC. Similar to the other experiments, animals were divided in two groups: ligated (n = 5) and sham (n = 5). Under ketamine (100 mg/kg) and acepromazine (0.5 mg/kg ip) anesthesia, a catheter (polyethylene-50 tubing) was implanted in the left carotid artery for mean arterial pressure monitoring. Blood flow measurements were performed continuously, as described previously, with minor modifications (28). Briefly, from a midabdominal incision, the left iliac artery was carefully isolated, and a small perivascular flow sensor (0.7 V) was placed around the artery and connected to a flowmeter (AT206, Transonic Systems, Ithaca, NY). Iliac vascular conductance (IVC) was calculated by dividing femoral blood flow by blood pressure. During the 6-min IPC bout, IVC was determined by averaging the responses during the first and last minutes of compressions.
Statistical analysis.
All data are presented as means ± SE. The ΔΔCt values were used for the statistical analysis of gene expression (mRNA) data (45). To facilitate interpretation of the data, mRNA levels are expressed as 2−ΔΔCt (fold change from control). Comparisons within each group (sham and ligated) and at each time (0 min and 150 min) for mRNA data were made using one-sample t-tests. The treatments (120 mmHg 2 s/2 s and 120 mmHg 4 s/16 s) were compared with each other using a two-sample t-test. To compare the groups (sham vs. ligated), the Cochran-Mantel-Haenszel methodology was employed. Immunohistochemistry results and hemodynamics were compared using ANOVA. In view of the large number of pair-wise comparisons being made, the comparison-wise error rate was controlled at the 0.01 level.
RESULTS
Skeletal muscle mRNA levels.
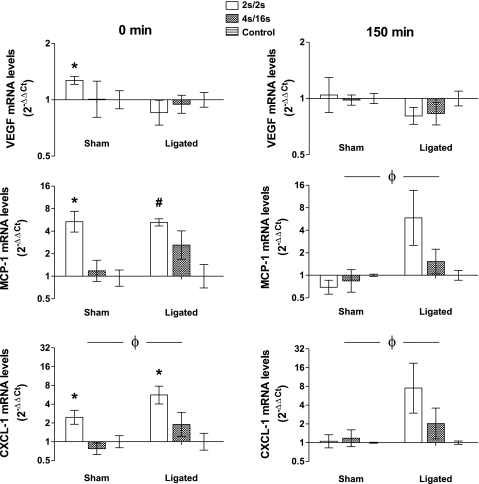
Relative mRNA expression of VEGF, MCP-1, and CXCL1 after IPC application is shown in Fig. 1. In the sham animals, high-frequency IPC (2 s/2 s) evoked a modest increase in VEGF mRNA that was only evident in the samples harvested immediately after the end of the compression session (0 min) (P = 0.015). In contrast, no changes in the expression of this gene were observed in the ligated animals at both time points. MCP-1 mRNA was robustly upregulated in the animals exposed to the high-frequency (2 s/2 s) protocol both in sham (P = 0.013) and ligated (P = 0.0007) animals at 0 min. On the other hand, distinct profiles in expression of MCP-1 were seen between sham and ligated animals at 150 min (P = 0.027). In the ligated group, MCP-1 mRNA levels tended to increase further after 150 min in the high-frequency group, while it returned to control levels in the sham animals. A similar pattern was seen for CXCL1. In the ligated group, CXCL1 mRNA expression in the high-frequency (2 s/2 s)-treated animals increased by ∼5-fold (P = 0.013) at 0 min and by ∼8-fold after 150 min, although the change in later time point was not statistically significant (P = 0.11). Compared with the sham group, ligated animals tended to have a different pattern of expression of CXCL1 at both 0 min (P = 0.02) and 150 min (P = 0.04).
Fig. 1.
Real-time RT-PCR of selected genes in skeletal muscle samples harvested immediately at the end (0 min) or 150 min following the completion of a single session of intermittent pneumatic leg compressions (IPC). Data are expressed as 2−ΔΔCt, normalized to GAPDH and relative to the average of the control group. Data are expressed as means ± SE; n = 4 in each treatment #P < 0.01 and *0.01 ≤ P ≤ 0.05 vs. control group. ϕP < 0.05 sham vs. ligated.
Immunohistochemistry.
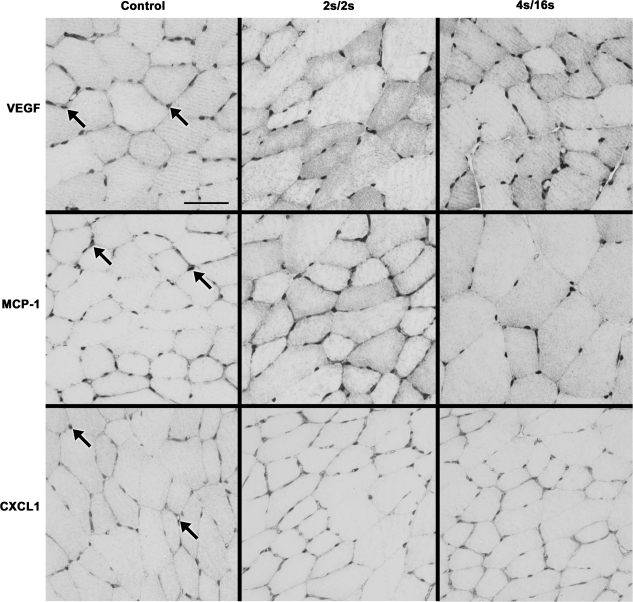
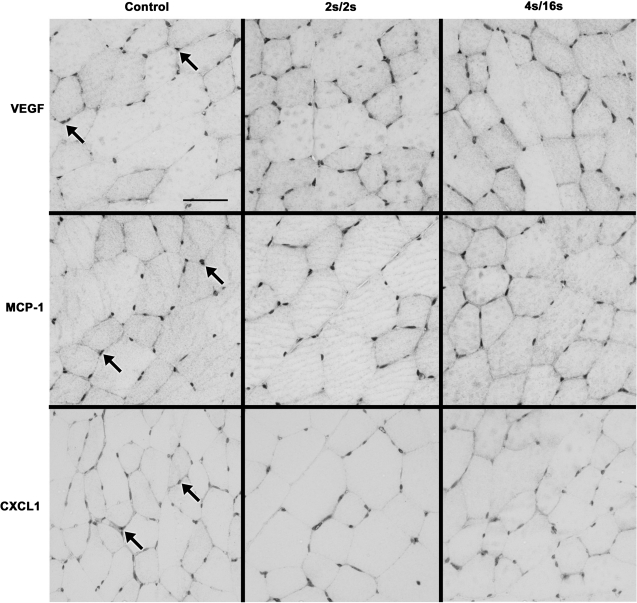
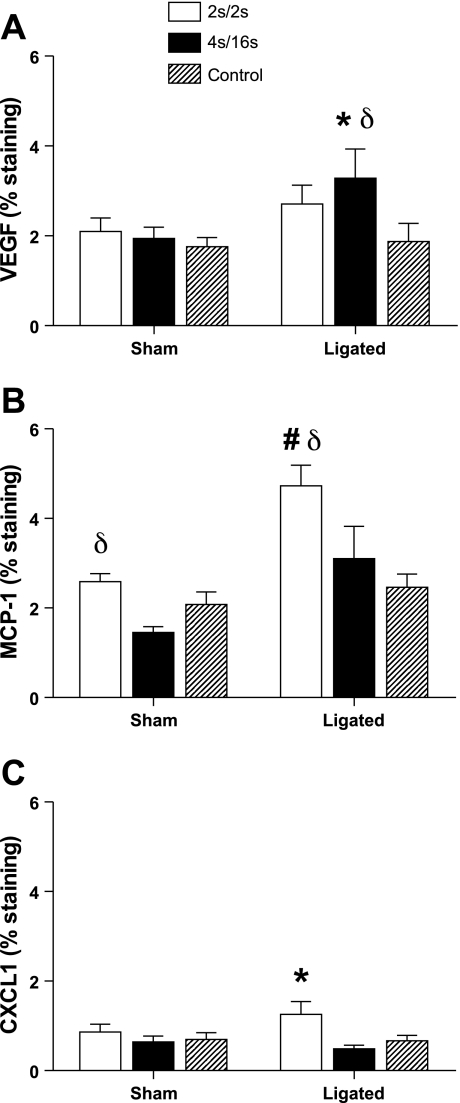
In the tissues harvested at 150 min after IPC, positive staining was mostly evident in the endomysium for all three factors (Figs. 2 and 3). Immunohistochemistry quantification is displayed on Fig. 4. In accordance with trends observed for mRNA changes, positive staining for MCP-1 in the muscle of ligated animals was significantly increased in the high-frequency group compared with the low-frequency (P = 0.012) and control (P < 0.01) groups (Fig. 4). Ligated animals exposed to high- and low-frequency IPC also had significantly increased percentage of MCP-1 staining compared with the same groups in the sham condition (P < 0.01). Immunostaining for CXCL-1 in ligated animals was found to be modestly increased in the high-frequency group compared with low-frequency (P = 0.01) and control (P = 0.04) groups. Interestingly, we also observed a minor increase in the amount of VEGF staining in the ligated animals that was especially evident in the low-frequency group compared with the controls (P = 0.018).
Fig. 2.
Representative images of immunostaining for VEGF (top), MCP-1 (middle), and CXCL1 (bottom) in cross sections of the rat tibialis anterior (TA) muscle harvested 150 min following the completion of a single session of IPC in the ligated animals. Arrows show regions of positive staining for the respective antibodies. Scale bar: 50 μm.
Fig. 3.
Representative images of immunostaining for VEGF (top), MCP-1 (middle), and CXCL1 (bottom) in cross sections of the rat TA muscle harvested after 150 min following the completion of a single session of IPC in sham-operated animals. Arrows show regions of positive staining for the respective antibodies. Scale bar: 50 μm.
Fig. 4.
Immunohistochemistry quantification in cross sections of the rat TA muscle harvested 150 min following the completion of a single session of IPC. % Staining is percent of slide area positive for staining. A: VEGF. B: MCP-1. C: CXCL1. Values are expressed as means ± SE; n = 4 in each treatment. #P < 0.01 and *0.01≤ P ≤ 0.05 vs. control group. δ0.01 ≤ P ≤ 0.05 vs. corresponding treatment in the sham animals.
Isolated perforating artery mRNA levels.
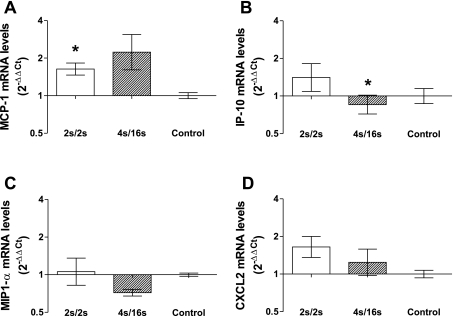
Figure 5 shows the mRNA expression of MCP-1, interferon gamma-induced protein 10 (IP-10), macrophage inflammatory protein-1α (MIP1-α), and chemokine (C-X-C motif) ligand 2 (CXCL2) in the perforating artery isolated from ligated animals 150 min following completion of the IPC session. MCP-1 expression increased in the high-frequency (P = 0.02) group compared with controls (Fig. 5A). A modest upregulation of CXCL2 mRNA was also observed in the high-frequency-treated group (P = 0.08) (Fig. 5D). In sharp contrast, no significant changes in the expression of these factors were detected in the arteries isolated from sham animals (data not shown). Interestingly, the expression of IP-10 was slightly decreased in the high-frequency group compared with controls (P = 0.03, Fig. 5B). No significant changes were observed in MIP-1α expression in both groups.
Fig. 5.
Real-time RT-PCR of MCP-1 (A), interferon gamma-induced protein (IP-10) (B), macrophage inflammatory protein 1α (MIP1-α) (C), and chemokine (C-X-C motif) ligand 2 (CXCL2) (D) in isolated samples of the rat perforating artery harvested 150 min following the completion of a single session of IPC. Data are expressed as 2−ΔΔCt, normalized to GAPDH and relative to the average of the control group. Data are expressed as means ± SE; n = 4 in each treatment. *0.01≤ P ≤ 0.05 vs. control.
Hemodynamic responses.
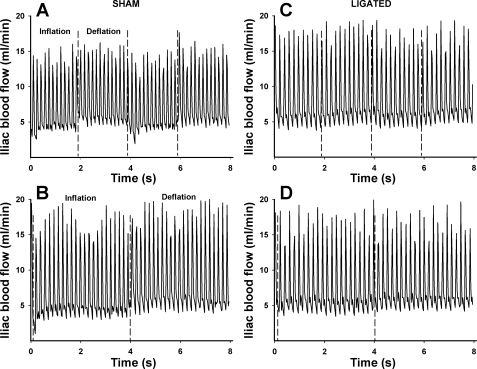
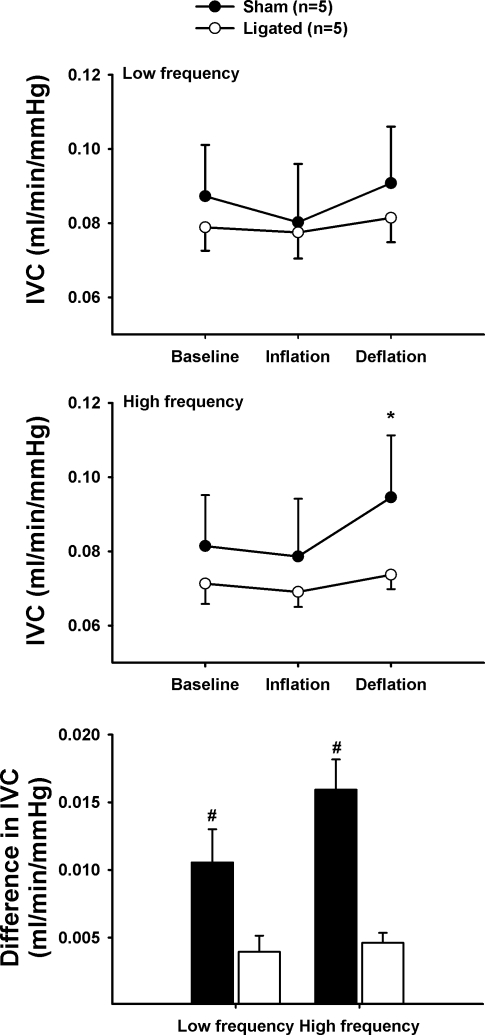
Representative traces of the blood flow responses to IPC are shown in Fig. 6. Intermittent compressions induce an oscillatory profile in blood flow characterized by a reduction during the cuff inflation phase followed by a hyperemic response during deflation (Fig. 6). Mean arterial pressure and average blood flow responses to this protocol are summarized in Table 2. IVC changes are depicted in Fig. 7. As seen in the blood flow tracings, it is evident that the magnitude of changes in IVC evoked by IPC is greatly diminished in ligated animals compared with nonligated controls both at low and high frequencies (Figs. 6 and 7). This is revealed by the calculation of the difference between average conductance during the deflation and inflation phases (Fig. 7C). On average, the change in conductance triggered by cyclic compressions was ∼217% greater in the sham animals compared with the ligated counterparts (P < 0.01).
Fig. 6.
Representative original tracings of the iliac blood flow responses to IPC applied at high (A and C) and low (B and D) frequencies.
Table 2.
Mean arterial blood pressure and blood flow in the iliac artery during IPC application
| Sham |
Ligated |
|||
|---|---|---|---|---|
| Low Frequency | High Frequency | Low Frequency | High Frequency | |
| Mean blood pressure, mmHg | ||||
| Baseline | 93.9 ± 7.6 | 98.4 ± 8.8 | 96.3 ± 2.6 | 101.6 ± 3.9 |
| Inflation | 97.3 ± 7.9 | 103.6 ± 9.6 | 103.1 ± 5 | 111.1 ± 3.5 |
| Deflation | 96.5 ± 8.0 | 103.0 ± 9.6 | 101.7 ± 4.7 | 111.4 ± 3.5 |
| Iliac blood flow, ml/min | ||||
| Baseline | 7.9 ± 1.0 | 7.8 ± 1.2 | 7.5 ± 0.7 | 7.2 ± 0.3 |
| Inflation | 7.4 ± 1.1 | 7.8 ± 1.2 | 7.8 ± 0.9 | 7.8 ± 0.3 |
| Deflation | 8.4 ± 1.1 | 9.4 ± 1.3 | 8.2 ± 0.9 | 8.2 ± 0.3 |
Values are expressed as means ± SE.
Fig. 7.
Effects of a short session of IPC application on iliac vascular conductance (IVC) responses. Top: low-frequency (4 s/16 s). Middle: high-frequency IPC (2 s/2 s). Bottom: difference in IVC between the deflation and inflation phases in sham (solid bars) and ligated (open bars) animals. *P < 0.01 vs. baseline and #P < 0.01 vs. ligated.
DISCUSSION
This is the first study to examine the impact of IPC application on skeletal muscle and isolated artery gene expression in a preclinical model of peripheral artery insufficiency. Our major finding was that a single session of IPC increases the expression of inflammatory chemokines in skeletal muscle and isolated collateral arteries in the ischemic hindlimbs of these animals. Of note, these changes in gene expression were especially evident in animals exposed to a protocol that employs a higher frequency of compressions (15 compressions/min) than the one commonly used in the clinical scenario (3 compressions/min). Together, these findings shed new light on the mechanistic basis of IPC therapy and most importantly, highlight the possibility that the protocols currently in use might be less than optimal for patients with claudication.
Methodological considerations.
The available clinical trials in stable claudicants have consistently shown positive effects of IPC therapy on exercise capacity and limb hemodynamics in these patients (5, 9, 17, 27). To be able to make meaningful insights into the mechanistic basis of this therapy using preclinical models, it is imperative that the benefits observed clinically are also detectable in the animal model. In that regard, we have recently documented in a sham-controlled study that as little as 2 wk of IPC therapy in a rat model of peripheral artery insufficiency enhanced muscle performance in situ, and blood flow to the plantaris muscle (blood flow to this muscle is dependent on the collateral circuit) (Roseguini BT, Arce-Esquivel AA, Newcomer SC, Yang HT, Terjung R, and Laughlin MH, unpublished observations). Therefore, these results indicate that the rat model employed here is reliable for the exploration of the molecular responses to this novel therapeutic approach and that our findings hold the potential to be translated to the clinical settings.
Collateral artery gene expression.
A major therapeutic goal of any intervention for patients with peripheral arterial disease is to restore the capacity for blood flow and oxygen delivery to the tissues located downstream from the arterial occlusion site(s). The growth and remodeling of collateral arteries are undoubtedly the most efficient ways of accomplishing this goal, and not surprisingly, several efforts have been made to design therapies that can modulate collateral arteriogenesis (41). Compression therapy has recently gained attention as a possible simple strategy to promote collateralization in these patients (6). In fact, animal (39) and clinical studies (38) have documented that long-term IPC therapy seems to enhance the density of collateral vessels in the lower limb. These findings prompted us to explore the possible mechanisms and molecular players involved in triggering these adaptations in the collateral network. The strategy employed here was to investigate gene expression responses to a single session of IPC in collateral arteries. This approach offers a unique opportunity to identify factors that are responsive to the therapy and that can ultimately play a role in the long-term remodeling process. On the basis of this design, we decided to target factors that are especially relevant to the early phases of collateral remodeling and that could respond in the time frame employed in this study. Accordingly, we selected a subset of four chemokines that have been recently shown to be among the most upregulated genes in the very early phase of the collateral remodeling process (4, 10).
Of the four chemokines studied, MCP-1/Ccl2 had the most consistent increase in its expression in response to IPC. This is a key observation given the prominent role of this factor for collateral artery expansion (12, 16, 22, 42). Indeed, a number of studies have clearly shown that upregulation of MCP-1 and its effects on monocyte recruitment seem to be fundamental for normal perfusion restoration after experimental arterial occlusion (12, 14, 42). This notion is strengthened by the observations that 1) MCP-1 is one of the most robustly upregulated genes in growing collaterals (4, 10), 2) local infusion of MCP-1 enhances collateral growth (16), and 3) mice lacking the receptor for MCP-1 (CCR2) have impaired arteriogenesis after arterial occlusion (12). Thus, albeit modest, IPC-induced increases in MCP-1 expression in isolated collaterals detected in the present investigation (Fig. 5) indicate that this factor could be involved in the documented IPC-mediated expansion of the collateral network (39).
The mechanisms by which IPC can alter MCP-1 expression in collateral arteries and promote arteriogenesis are unclear, but it has been suggested that temporary cyclic increases in flow and shear stress induced by this therapy could play a role (6). To gain insights into this issue, we performed experiments to assess the hemodynamic responses in the iliac arteries, which are located upstream from the collateral vessels, before and during a short session of IPC application. We observed that IPC caused only a minor, nonsignificant increase in iliac vascular conductance during the deflation phase of the cycle in the ligated animals (Figs. 6 and 7). Although these observations do not preclude the notion that such small changes in flow could trigger MCP-1 upregulation in collateral arteries, it is tempting to suggest that additional factors play a major role. One possible candidate is the circumferential stretch caused by intermittent changes in pressure in the vascular network. It is conceivable that the rapid and robust compressions of the calf evoke changes in pressure and circumferential strain in the upstream arterial network, thereby promoting upregulation of arteriogenic factors. Lending support to this hypothesis, studies by Demicheva et al. (10) indicated that cyclic stretch, but not shear stress, is responsible for MCP-1 upregulation during arteriogenesis. These interesting findings encourage future studies to determine the magnitude of changes in pressure in the collateral network during IPC application.
Skeletal muscle responses to IPC.
The rationale for studying IPC-induced gene expression changes in skeletal muscle is based on the notion that mechanical forces can promote vascular remodeling and changes in muscle phenotype (2). Since IPC is known to cause pronounced changes in intramuscular pressure (29) and blood flow (40), we reasoned that one session of this therapy could also alter gene and protein expression in the compressed tissue. In previous studies in healthy rats, we documented that a single session of IPC increases VEGF and MCP-1 mRNA expression in the TA muscle (28). Here, we report for the first time that IPC application induces a potent upregulation of MCP-1 and CXCL-1 mRNA and protein expression, as well as discrete changes in VEGF protein expression in the TA of rats with peripheral artery insufficiency.
Another fundamental observation in these studies is that the pattern of changes in gene and protein expression evoked by IPC differed in many ways between sham and ligated animals. As opposed to the short-lived increase in mRNA expression of VEGF, MCP-1, and CXCL1 and lack of accompanying changes in the corresponding proteins in sham animals, IPC evoked a sustained increase in MCP-1 and CXCL1 expression in ligated rats (Figs. 1–4). The lack of pronounced changes in iliac vascular conductance during IPC in ligated animals compared with sham controls (Figs. 6 and 7) would suggest that factors other than blood flow are responsible for the difference in the expression of these chemokines between the two groups. However, animals with peripheral artery insufficiency have a vast array of changes in skeletal muscle phenotype and gene expression (24, 25), and it is conceivable that these minor, episodic changes in flow during IPC combined with the hyperemia experienced at the end of the period of application triggers a more powerful inflammatory response than that observed in nonligated controls.
These marked changes in chemokine expression could affect several remodeling processes in the muscle. For example, it is known that besides promoting arteriogenesis, MCP-1 is also a powerful angiogenic factor (15, 30, 44) and participates directly in muscle repair by recruiting inflammatory mediators and myogenic cells (36). Likewise, CXCL1 has strong angiogenic properties (33) and has been shown to be involved in homing of bone marrow angioblasts and neovascularization in the ischemic heart (18). Together, upregulation of these and other chemokines during IPC could possibly aid in the functional recovery of the muscle following the ischemic insult (31) and potentially promote angiogenesis in the ischemic compressed tissue.
Effect of compression frequency.
A key finding of the present study was that changes in gene and protein expression in response to IPC therapy appear to be modulated by compression frequency. In accordance with our previous report in healthy animals (28), it is clear that IPC applied at a high frequency (15 cycles/min) evokes more consistent and robust effects in gene and/or protein expression compared with a lower frequency (3 cycles/min). In our view, this finding holds promising clinical application given that the currently available devices designed to treat patients with intermittent claudication operate only at low frequencies. The reasons behind the choice of an operating frequency of three compressions/minute with 17–18 s of interval between each cycle in most commercially available devices are unknown. These pumps have been built on the premise that repeated increases in arterial inflow, triggered by the enhanced arterial-venous pressure gradient during compression, are the major mechanisms of action of IPC (7, 8). In theory, however, a shorter interval between compressions should keep venous pressures at lower levels throughout the stimulation period, thereby magnifying the hyperemic response to each compression cycle. Further, the current design ignores the possibility that factors other than increased blood flow could be involved in promoting the beneficial adaptations induced by this therapy. If, as discussed above, increased circumferential stretch in collateral arteries and repeated forceful compressions of the muscles are also important, it would be appropriate to select a frequency that enhances the action of these mechanisms. Along these lines, alternative compression strategies that employ much higher frequencies, such as enhanced external counterpulsation, have begun to be tested in cohorts of patients with claudication (1, 35). These initial studies indicate that the extremely high frequencies adopted in these devices are safe and seem to be associated with improved outcomes in these patients (35). Therefore, it is conceivable that long-term IPC at a higher frequency than currently employed could magnify the established positive clinical results. To date, however, no study has evaluated the impact of compression frequency on clinically relevant outcomes in claudicants.
Limitations.
A major limitation of studying gene/protein expression responses to a single session of IPC therapy is that this approach does not offer insights into the temporal profile and direct functional consequences associated with a repeated, long-term intervention. We selected a few targets that are known to be associated with inflammation and vascular remodeling and reasoned that determining the acute responses of these genes to IPC therapy could give us some clues about the molecular players involved in the chronic adaptations to this therapy. It should be recognized, however, that this reductionist strategy is limited, and other undetermined factors could play a role in the adaptive process. Future studies should, therefore, employ more in-depth approaches, such as whole-genome transcriptional profiling, to identify more candidates that ultimately determine the functional benefits associated with this novel therapy.
Conclusions.
In conclusion, IPC application acutely increased the expression of factors involved in vascular remodeling in skeletal muscle and isolated collateral arteries. The magnitude and temporal profile of these responses appear different in a model of hindlimb ischemia compared with healthy controls. Most importantly, a compression frequency higher (15 cycles/min) than the one commonly employed in commercial devices (3 cycles/min) seems to evoke greater and more consistent effects in the expression of these factors. These findings encourage future translational studies aimed at finding optimal IPC stimulation characteristics for claudicants.
GRANTS
This research was supported by NIH Grants RR-18276, HL-36088 and a Doctoral Student Research Grant from the American College of Sports Medicine Foundation (to B. Roseguini). Bruno Roseguini is a Fulbright/Brazilian Ministry of Education (Capes) fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Ann Melloh, Jennifer Casati, and Alexa Bermudez for their invaluable technical assistance and Dr. Richard Madsen for assistance with the statistical analysis. We are also indebted to Dr. Steve Yang for critical insights in the study design and Dr. Ronald Terjung for allowing a portion of the data collection to be carried out in his laboratory.
REFERENCES
- 1. Bondke Persson A, Buschmann EE, Lindhorst R, Troidl K, Langhoff R, Schulte KL, Buschmann I. Therapeutic arteriogenesis in peripheral arterial disease: combining intervention and passive training. Vasa 40: 177–187, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Campbell SJ, Wilcockson DC, Butchart AG, Perry VH, Anthony DC. Altered chemokine expression in the spinal cord and brain contributes to differential interleukin-1beta-induced neutrophil recruitment. J Neurochem 83: 432–441, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106: 1870–1881, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Haro J, Acin F, Florez A, Bleda S, Fernandez JL. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 51: 857–862, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Delis KT. The case for intermittent pneumatic compression of the lower extremity as a novel treatment in arterial claudication. Perspect Vasc Surg Endovasc Ther 17: 29–42, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Delis KT, Azizi ZA, Stevens RJ, Wolfe JH, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower-limb venous emptying. Eur J Vasc Endovasc Surg 19: 261–269, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Delis KT, Knaggs AL. Duration and amplitude decay of acute arterial leg inflow enhancement with intermittent pneumatic leg compression: an insight into the implicated physiologic mechanisms. J Vasc Surg 42: 717–725, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 241: 431–441, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res 103: 477–484, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res 95: 449–458, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res 94: 671–677, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. Am J Physiol Heart Circ Physiol 283: H2012–H2020, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hoefer IE, Grundmann S, van Royen N, Voskuil M, Schirmer SH, Ulusans S, Bode C, Buschmann IR, Piek JJ. Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 181: 285–293, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 105: 1405–1407, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 80: 829–837, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 30: 164–175, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowski P, Boyle A, Homma S, Wang SF, Itescu S. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol 40: 455–464, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol 284: H1668–H1678, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Neto JS, Nakao A, Toyokawa H, Nalesnik MA, Romanosky AJ, Kimizuka K, Kaizu T, Hashimoto N, Azhipa O, Stolz DB, Choi AM, Murase N. Low-dose carbon monoxide inhalation prevents development of chronic allograft nephropathy. Am J Physiol Renal Physiol 290: F324–F334, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Niiyama H, Kai H, Yamamoto T, Shimada T, Sasaki K, Murohara T, Egashira K, Imaizumi T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol 44: 661–666, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Park Y, Hirose R, Dang K, Xu F, Behrends M, Tan V, Roberts JP, Niemann CU. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery 143: 243–251, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg 41: 481–489, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg 42: 101–112, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Ramaswami G, D'Ayala M, Hollier LH, Deutsch R, McElhinney AJ. Rapid foot and calf compression increases walking distance in patients with intermittent claudication: results of a randomized study. J Vasc Surg 41: 794–801, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol 298: H1991–H2000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roseguini BT, Sheldon R, Stroup A, Bell JW, Maurer D, Crist BD, Laughlin MH, Newcomer SC. Impact of chronic intermittent external compressions on forearm blood flow capacity in humans. Eur J Appl Physiol 111: 509–519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96: 34–40, 2000 [PubMed] [Google Scholar]
- 31. Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81: 775–785, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med 347: 1941–1951, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 16: 593–609, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Tan X, Qi WN, Gu X, Urbaniak JR, Chen LE. Intermittent pneumatic compression regulates expression of nitric oxide synthases in skeletal muscles. J Biomech 39: 2430–2437, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Thakkar BV, Hirsch AT, Satran D, Bart BA, Barsness G, McCullough PA, Kennard ED, Kelsey SF, Henry TD. The efficacy and safety of enhanced external counterpulsation in patients with peripheral arterial disease. Vasc Med 15: 15–20, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298: R1173–R1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valles A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis 22: 312–322, 2006 [DOI] [PubMed] [Google Scholar]
- 38. van Bemmelen P, Char D, Giron F, Ricotta JJ. Angiographic improvement after rapid intermittent compression treatment [ArtAssist] for small vessel obstruction. Ann Vasc Surg 17: 224–228, 2003 [DOI] [PubMed] [Google Scholar]
- 39. van Bemmelen PS, Choudry RG, Salvatore MD, Goldenberg M, Goldman BI, Blebea J. Long-term intermittent compression increases arteriographic collaterals in a rabbit model of femoral artery occlusion. Eur J Vasc Endovasc Surg 34: 340–346, 2007 [DOI] [PubMed] [Google Scholar]
- 40. van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ, Ramsey DE, Sumner DS. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 19: 1052–1058, 1994 [DOI] [PubMed] [Google Scholar]
- 41. van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol 55: 17–25, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med 9: 287–292, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells : implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol 19: 2085–2093, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]