Abstract
Objective
The aim was to describe a case of hypersensitivity to rabbit antithymocyte globulin (rATG) occurring in the context of islet transplantation.
Methods
A 36-year-old woman with type 1 diabetes was admitted for islet transplantation. rATG was administered the first day (1.5 mg/kg) with methylprednisolone (2 mg/kg), and on the second day (1.5 mg/kg) without glucocorticoid to avoid potential toxicity to the anticipated islet transplant.
Results
At the end of the rATG infusion on the second day she developed hives over her face, chest, and back and tender erythema at her intravenous site (Arthus reaction). Islet transplantation was not performed. She reported exposure to a pet rabbit for 2 years in childhood. Overnight, fever developed and the rash evolved into an erythematous morbilliform eruption affecting the torso. Serum high-sensitivity C-reactive protein (hsCRP) and the erythrocyte sedimentation rate (ESR) were elevated; serum complements C3 and C4 were normal. She received prednisone (50 mg) with subsequent resolution of the rash. Nine days after her initial reaction, she developed a recurrence of the rash and fever with arthralgias; levels of C3 and C4 had fallen. Methylprednisolone (125 mg, twice) was required for symptom improvement, and was gradually tapered as prednisone over the next 4 weeks with resolution of the complement, ESR, and hsCRP abnormalities. Five months after the initial attempt at islet transplantation, she returned to receive 7,879 IE/kg via portal vein infusion under basiliximab, etanercept, tacrolimus, and sirolimus immunosuppression and has required no to low-dose (0.1 U/kg/d) insulin to maintain near-normal glycemic control for > 12 months after transplantation.
Conclusions
Our patient’s initial hypersensitivity reaction to rATG was followed by immune-complex type 3 hypersensitivity (serum sickness) requiring high-dose glucocorticoids. Canceling the initial islet infusion proved to be wise, and the patient subsequently did well with islet transplantation under an alternative induction agent.
The introduction of steroid-free immunosuppression for islet transplantation with the Edmonton protocol was a major advance enabling reproducible independence from insulin therapy for type 1 diabetic recipients, albeit with the requirement for islets isolated from a median of 2 donor pancreata.1 More recent work from Minneapolis incorporating a regimen of rabbit antithymocyte globulin (rATG) at the time of induction with similar low-dose calcineurin inhibitor and mammalian target of rapamycin inhibitor maintenance therapy, as in the Edmonton protocol, appears to have improved rates of insulin independence, more frequently with islets isolated from a single donor,2 and sustained for a longer time.3 This regimen of rATG is presently being evaluated as part of the multicenter Clinical Islet Transplantation Consortium Protocol (CIT07).4
Heterologous serum products of polyclonal immunoglobulin G, such as ATG, have been associated rarely with immediate type 1,5 and more often with immune-complex type 3 (serum sickness), hypersensitivity reactions, particularly to products derived from horses,6 but also to those derived from rabbits,7 presumably owing to prior sensitization to the particular animal species.8 Although earlier reports estimated the incidence of serum sickness ~8.5% with rATG,7 a more recent estimate with the use of a lower-dose (total 6 mg/kg) rATG regimen is ~0.25%.8 We describe a case of immune-complex hypersensitivity to rATG occurring in the context of islet transplantation according to the CIT07 protocol.
CASE REPORT
A 36-year-old woman with type 1 diabetes was admitted for islet transplantation. rATG was administered the first day at (0.5 mg/kg), with methylprednisolone (1 mg/kg) before and midway through the infusion, followed by 1.0 mg/kg, and on the second day at 1.5 mg/kg without further glucocorticoid to avoid potential toxicity to the anticipated islet transplant. At the end of the rATG infusion on the second day, the patient developed hives over her face, chest, and back and tender erythema at her intravenous catheter site. She was treated with diphenhydramine and hydrocortisone which resulted in only modest improvement, so the scheduled islet transplant was canceled. She reported having a pet rabbit when she was 10–12 years old, but no known allergy to rabbits. Overnight, her temperature increased to 100.6°F and the rash evolved into an erythematous morbilliform eruption affecting the torso (Fig 1A). Serum high-sensitivity C-reactive protein (hsCRP) was elevated at 178.4 mg/L (normal, <7.4 mg/L), as was the erythrocyte sedimentation rate (ESR) at 28 mm/h (normal, <25 mm/h); serum C3 and C4 were normal (Fig 2). She received prednisone (50 mg) with subsequent resolution of the rash (Fig 1B) and completed 3 more 30 mg doses.
Fig 1.
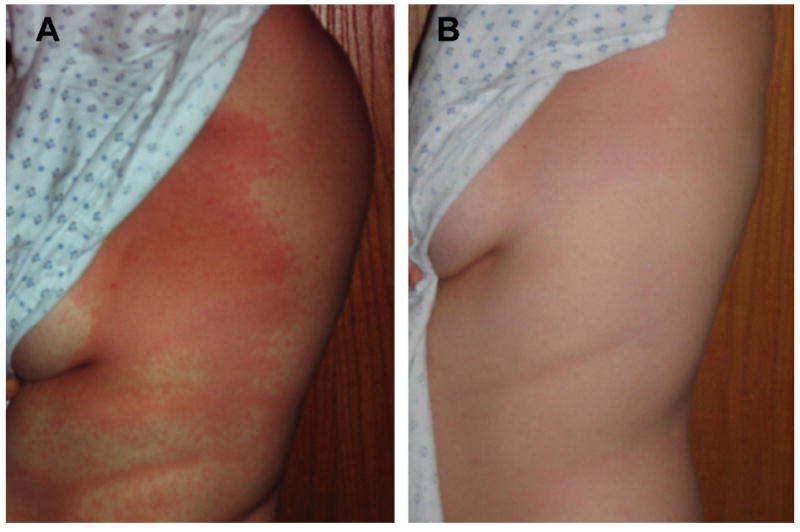
(A) Erythematous morbilliform eruption affecting the torso 2 days after initial exposure to rATG and 1 day after rATG was discontinued due to urticaria and an Arthus reaction present at the intravenous infusion site. (B) Complete resolution of the rash a few hours after receiving 50 mg prednisone orally.
Fig 2.
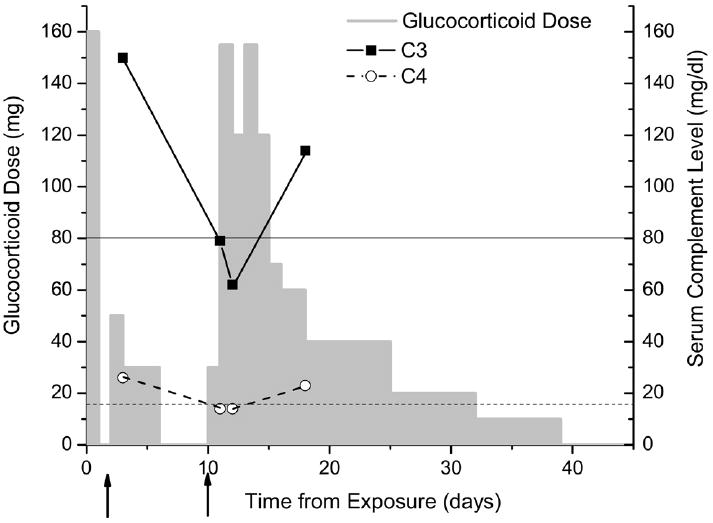
Glucocorticoid dose and serum complement levelsl during the course of the rATG hypersensitivity. The first arrow indicates the onset of the initial presentation with urticaria and Arthus reaction, and the second arrow indicates the onset of the recurrent rash accompanied with arthralgias. At the time of the hypersensitivity recurrence, serum complement levels had fallen below normal for both C3 (lower limit of normal indicated by thin solid line) and C4 (lower limit of normal indicated by thin dashed line), identifying the reaction as immune-complex mediated (serum sickness).
Nine days after her initial reaction, she developed a recurrence of the rash and despite resuming prednisone as 30 mg twice daily, her temperature increased to 100.4°F accompanied with arthralgias; serum levels of C3 and C4 had fallen (Fig 2). Methylprednisolone (125 mg, twice) was required for symptom resolution, and it was followed at 40 mg every 8 hours for 4 doses before tapering as prednisone over the next 4 weeks. One week after the second reaction, serum levels of C3 and C4 were normal (Fig 2), as was the ESR at 9 mm/h. The hsCRP level remained slightly elevated at 8.3 mg/L 6 weeks after exposure to rATG and finally became normal at 6.0 mg/L by 8 weeks. Rabbit epithelium IgE and IgG antibodies were negative both before the first dose of rATG and 8 weeks later, whereas rabbit serum protein IgG antibodies were negative before rATG and then positive at 34 μg/mL (normal, <7 μg/mL) 8 weeks after exposure.
Five months after the initial attempt at islet transplantation, while still T-lymphocyte depleted as a result of the earlier 3.0 mg/kg rATG (Fig 3), the patient returned to receive 7,879 islet equivalents per kg body weight via portal vein infusion under basiliximab (20 mg twice), etanercept (2), low-dose tacrolimus, and sirolimus immunosuppression. Before transplantation, the patient’s insulin requirements were 0.45 u/kg/d administered via continuous subcutaneous insulin infusion and the glycated hemoglobin value (HbA1c) ranged from 8.2% to 9.9%. At 2 months after transplantation, exogenous insulin therapy was tapered off, and since 9 months after transplantation she has resumed intermittent use of low-dose insulin (0.1 U/kg/d) as a once-daily subcutaneous injection of glargine; the HbA1c has ranged from 6.2% to 6.5% since her islet transplant.
Fig 3.
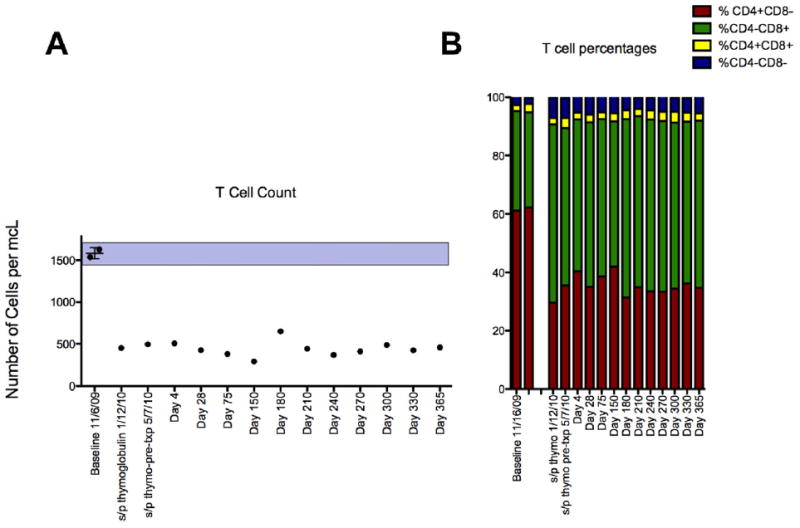
(A) T-lymphocyte cell counts in peripheral blood were markedly reduced after receiving 3.0 mg/kg rATG, an effect maintained in the absence of maintenance immunosuppression for > 5 months until a second attempt at islet transplantation was successful under basiliximab and etanercept induction, and then for another year under tacrolimus and sirolimus maintenance. (B) All T-lymphocyte cell subsets were similarly reduced following rATG.
DISCUSSION
Our patient experienced an immune-complex type 3 hypersensitivity (serum sickness) reaction that presented pathognomonically 10 days after exposure to rATG with rash, fever, and arthralgias that required high-dose glucocorticoids for control. Unique to this case was a presenting prodrome of urticaria, rash, and fever initially thought to represent an immediate type 1 hypersensitivity reaction that responded to moderate-dose glucocorticoids. However, this initial reaction did not occur with the first dose of rATG, as would be expected with immediate hypersensitivity (D.F.L., unpublished data), but after termination of methylprednisolone as a prophylactic medication in anticipation of the planned islet transplant. Subsequent testing for anti-rabbit antibodies demonstrated the development of serum IgG but no IgE immunoglobulin, supporting immune-complex rather than immediate hypersensitivity as the allergic mechanism.
Immune-complex hypersensitivity is mediated by IgG, which activates complement via the classic pathway producing the anaphylatoxins C3a and C5a that can lead to mast cell degranulation manifesting as urticaria, as seen early on in our patient. This process is likely unrecognized in the majority of transplant patients who develop serum sickness, because of the use of high doses of glucocorticoid as an immunosuppressive agent early after transplantation. We suspect that this early reaction required preformed IgG, resulting from having a pet rabbit that was below detection limits before rATG exposure, because the tender erythema that developed at the intravenous site coincident with the urticaria was highly reminiscent of the local Arthus reaction generated in animal models of serum sickness. By 10 days after exposure to rATG, IgG levels presumably had increased sufficiently to form complexes with the polyclonal rabbit IgG, depositing in the endothelium and fixing complement, as manifested by the recurrent rash, arthralgias, and decreased serum levels of complements C3 and C4.
We conclude from this case that the present practice of eliminating glucocorticoids at the time of islet transplantation may unmask hypersensitivity to rATG. Recognition of delayed urticaria or an Arthus reaction as a possible prodrome to serum sickness should help avoid the conduct of an islet transplant in a recipient soon to require highdose glucocorticoid therapy. As an alternative to glucocorticoid therapy, plasmapheresis may be considered.8,9 Canceling our patient’s initial islet infusion proved to be wise, and she subsequently did well with islet transplantation under an alternative induction agent.
Acknowledgments
Funding: This research was performed as a project of the Clinical Islet Transplantation Consortium, a collaborative clinical research project headquartered at the National Institute of Diabetes and Digestive and Kidney Diseases and National Institute of Allergy and Infections Diseases and supported by Public Health Services grant U01 DK070430 to A.N. from the National Institutes of Health. Also supported in part by Measey and Humpton Fellowship awards to S.A.S. and a Schiffrin award in autoimmune research to M.R.R. The authors are also indebted to Eileen Markmann, Maral Palanjian, and Cornelia Dalton-Bakes for their subject care and coordination of the clinical trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 3.Bellin MD, Kandaswamy R, Parkey J, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CIT. [April 26, 2011]; website: Available at http://www.citisletstudy.org/
- 5.Bielory L, Wright R, Nienhuis AW, Young NS, Kaliner MA. Antithymocyte globulin hypersensitivity in bone-marrow failure patients. JAMA. 1988;260:3164. [PubMed] [Google Scholar]
- 6.Bielory L, Gascon P, Lawley TJ, Young NS, Frank MM. Human serum sickness—a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone-marrow failure. Medicine. 1988;67:40. [PubMed] [Google Scholar]
- 7.Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: Case report and literature review. Liver Transplant. 2007;13:647. doi: 10.1002/lt.21098. [DOI] [PubMed] [Google Scholar]
- 8.Boothpur R, Hardinger KL, Skelton RM, et al. Serum sickness after treatment with rabbit antithymocyte globulin in kidney transplant recipients with previous rabbit exposure. Am J Kid Dis. 2010;55:141. doi: 10.1053/j.ajkd.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanriover B, Chuang P, Fishbach B, et al. Polyclonal antibody–induced serum sickness in renal transplant recipients: treatment with therapeutic plasma exchange. Transplantation. 2005;80:279. doi: 10.1097/01.tp.0000165093.13046.b3. [DOI] [PubMed] [Google Scholar]


