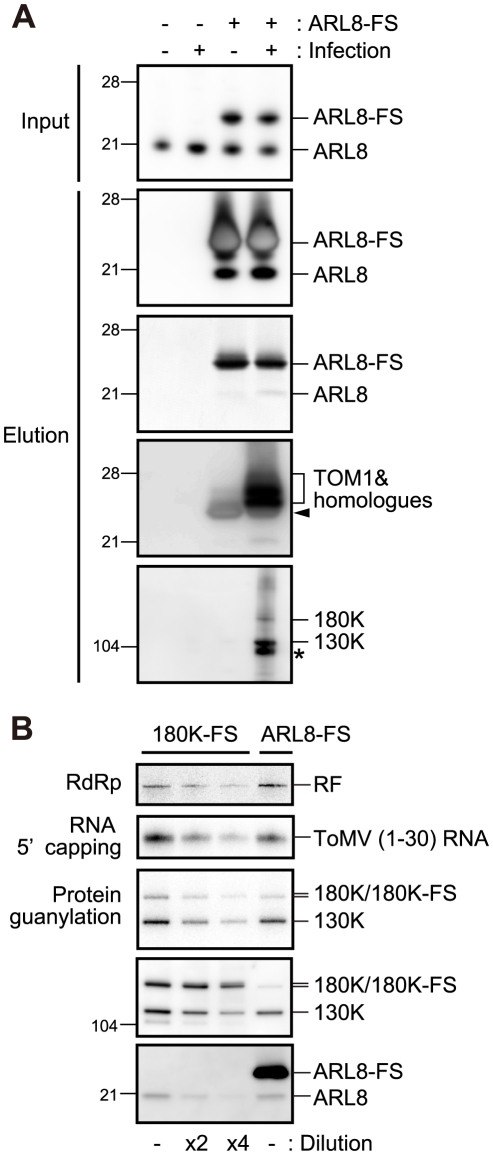
Figure 2. Affinity purification of ARL8-FS from uninfected and ToMV-infected BY-2 cells.
(A) Copurification of TOM1, 130K and 180K proteins with NtARL8a-FS. The P15 membrane fractions were prepared from the extracts of NtARL8a-FS-expressing BY-2 cells that had or had not been infected with ToMV-GFP. NtARL8a-FS was solubilized with LPC and immunopurified with the anti-FLAG antibody [27]. NtARL8a-FS and endogenous ARL8 proteins were detected by immunoblotting and Coomassie brilliant blue-staining of the blotted membrane (second and third panels from the top, respectively). TOM1 and ToMV 130K and 180K replication proteins were detected by immunoblotting. Control experiments with BY-2 cells that did not express NtARL8a-FS were performed in parallel. The arrowhead shows signals corresponding to the crossreaction of anti-TOM1 antibodies with NtARL8a-FS. The asterisk indicates a degradation product of the 130K/180K proteins. The positions of protein markers are shown on the left with their molecular weights (x10−3). (B) Copurification of ToMV RdRp and capping activities with ARL8-FS. The P15 membrane fractions were prepared from ARL8-FS-expressing and ToMV-GFP-infected BY-2 cells or from ToMV-180FS-GFP-infected BY-2 cells that were evacuolated. The fractions were solubilized with LPC and immunopurified with the anti-FLAG antibody [27]. The FLAG-purified fractions were subjected to RdRp, RNA 5′ capping, and protein guanylation assays. The fractions were also subjected to immunoblot analysis to detect ToMV replication proteins and ARL8. Where specified, the fraction was diluted 2- or 4-fold. The RdRp reaction was performed in the presence of [α-32P]CTP using exogenously added ToMV RNA as a template as described previously [27], and analyzed by PAGE. RNA 5′ capping and protein guanylation reactions were performed and products were analyzed as described in the Materials and Methods. 32P-labeled bands were detected with an image analyzer (BAS 2500, Fujifilm). The positions corresponding to double-stranded ToMV RNA (RF), ToMV (1–30) RNA and 130K, 180K (180K-FS), and ARL8 (ARL8-FS) are indicated on the right.