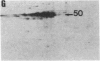
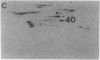
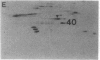
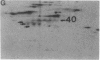
Abstract
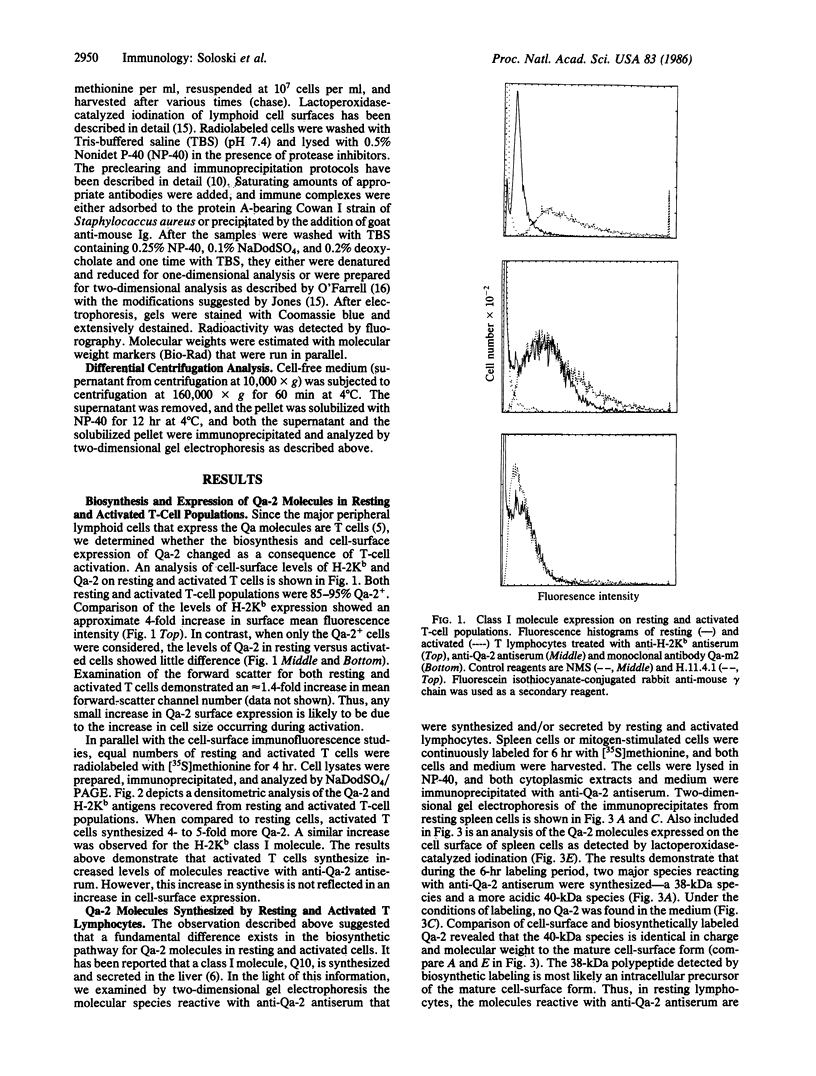
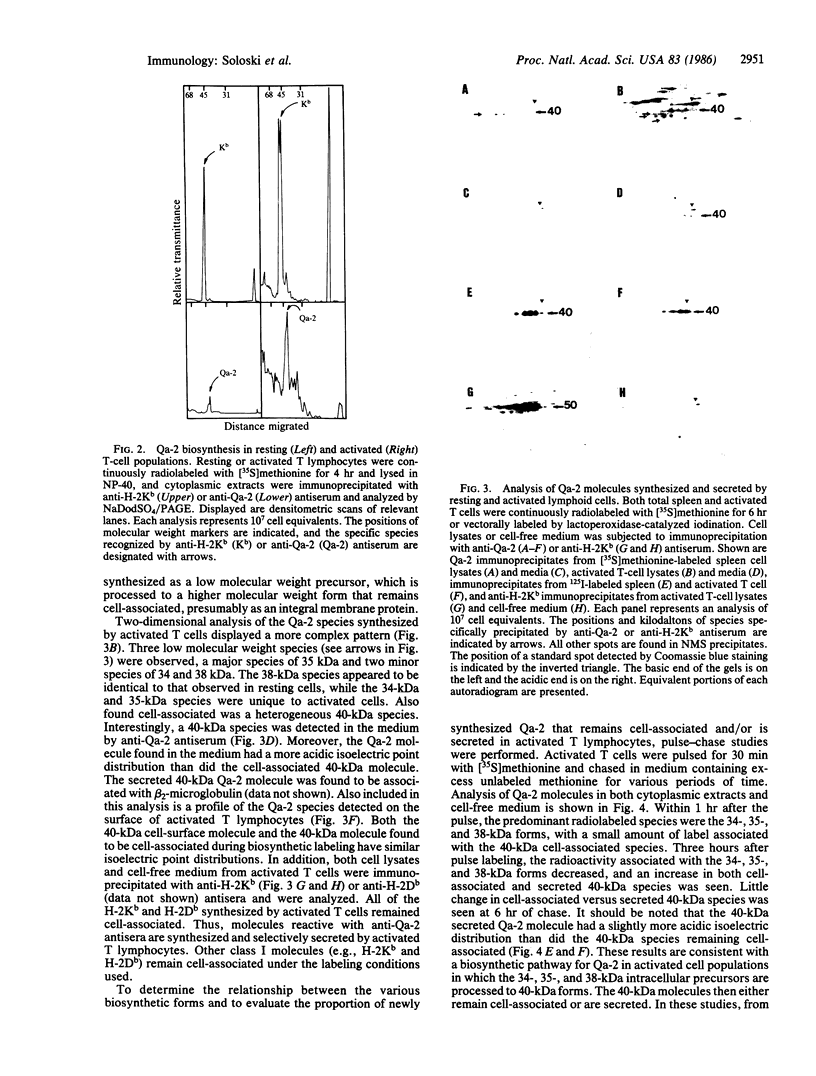
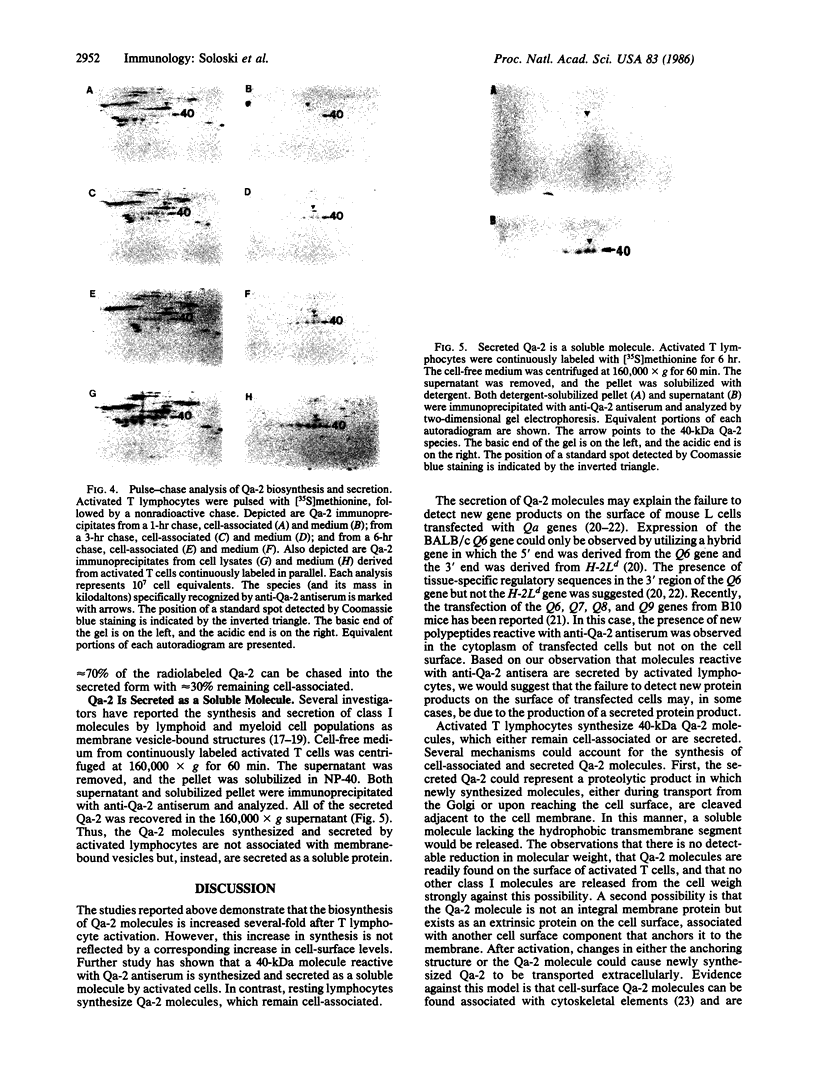
The biosynthesis and expression of the tissue-specific class I molecule Qa-2 have been studied in resting and activated T-cell populations. Polyclonal activation of T lymphocytes induces a 3- to 4-fold increase in the biosynthesis of Qa-2 molecules but no increase in cell-surface levels. Analysis of the biosynthetic pathway of the Qa-2 molecule in activated lymphocytes reveals that approximately equal to 70% of the newly synthesized Qa-2 molecules are secreted as soluble molecules. In resting-cell populations, Qa-2 remains entirely cell-associated. This process is unique to the Qa-2 molecule, since other class I molecules (e.g., H-2Kb and H-2Db) synthesized by activated cells remain cell-associated. The possibility that the secreted Qa-2 molecule is the product of a new Qa gene or an alternatively spliced mRNA is considered. These results indicate that the Qa-2 molecules may not just function as a cell-surface recognition structure but also may serve a role as a soluble factor synthesized by activated lymphoid cell populations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook R. G., Jenkins R. N., Flaherty L., Rich R. R. The Qa-1 alloantigens. II. Evidence for the expression of two Qa-1 molecules by the Qa-1d genotype and for cross-reactivity between Qa-1 and H-2K. J Immunol. 1983 Mar;130(3):1293–1299. [PubMed] [Google Scholar]
- Devlin J. J., Lew A. M., Flavell R. A., Coligan J. E. Secretion of a soluble class I molecule encoded by the Q10 gene of the C57BL/10 mouse. EMBO J. 1985 Feb;4(2):369–374. doi: 10.1002/j.1460-2075.1985.tb03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. G., Cone R. E. I-Kk and H-2Kk antigens are shed as supramolecular particles in association with membrane lipids. J Immunol. 1981 Aug;127(2):482–486. [PubMed] [Google Scholar]
- Emerson S. G., Murphy D. B., Cone R. E. Selective turnover and shedding of H-2K and H-2D antigens is controlled by the major histocompatibility complex. Implications for H-2-restricted recognition. J Exp Med. 1980 Oct 1;152(4):783–795. doi: 10.1084/jem.152.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli D., Rungger-Brändle E. Association of specific cell-surface glycoproteins with a triton X-100-resistant complex of plasma membrane proteins isolated from T-lymphoma cells (P1798). Exp Cell Res. 1985 Jan;156(1):239–250. doi: 10.1016/0014-4827(85)90278-2. [DOI] [PubMed] [Google Scholar]
- Hogarth P. M., Basten A., Prichard-Briscoe H., Henning M. H., Sutton V. R., McKenzie I. F. The distribution of the Qa-2 alloantigen on functional T lymphocytes. J Immunol. 1985 Sep;135(3):1632–1636. [PubMed] [Google Scholar]
- Hogarth P. M., Crewther P. E., McKenzie I. F. Description of a Qa-2 like alloantigen (Qa-m2). Eur J Immunol. 1982 May;12(5):374–379. doi: 10.1002/eji.1830120504. [DOI] [PubMed] [Google Scholar]
- Iványi D., Cherry M., Démant P. Molecular heterogeneity of D-end products detected by anti-H-2.28 sera. III. Reactivity of certain anti-H-2.28 alloantisera with Qa-2 antigen. Immunogenetics. 1982;15(5):477–484. doi: 10.1007/BF00345907. [DOI] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia antigens by two-dimensional polyacrylamide gel electrophoresis. Methods Enzymol. 1984;108:452–466. doi: 10.1016/s0076-6879(84)08111-8. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Koch G. L., Smith M. J. An association between actin and the major histocompatibility antigen H-2. Nature. 1978 May 25;273(5660):274–278. doi: 10.1038/273274a0. [DOI] [PubMed] [Google Scholar]
- Kress M., Glaros D., Khoury G., Jay G. Alternative RNA splicing in expression of the H-2K gene. Nature. 1983 Dec 8;306(5943):602–604. doi: 10.1038/306602a0. [DOI] [PubMed] [Google Scholar]
- Kress M., Glaros D., Khoury G., Jay G. Alternative RNA splicing in expression of the H-2K gene. Nature. 1983 Dec 8;306(5943):602–604. doi: 10.1038/306602a0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Peterson P. A. Isolation and partial characterization of a beta2-microglobulin-containing, H-2 antigen-like murine serum protein. Biochemistry. 1978 Oct 31;17(22):4794–4801. doi: 10.1021/bi00615a029. [DOI] [PubMed] [Google Scholar]
- Lalanne J. L., Cochet M., Kummer A. M., Gachelin G., Kourilsky P. Different exon-intron organization at the 5' part of a mouse class I gene is used to generate a novel H-2Kd-related mRNA. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7561–7565. doi: 10.1073/pnas.80.24.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne J. L., Transy C., Guerin S., Darche S., Meulien P., Kourilsky P. Expression of class I genes in the major histocompatibility complex: identification of eight distinct mRNAs in DBA/2 mouse liver. Cell. 1985 Jun;41(2):469–478. doi: 10.1016/s0092-8674(85)80020-9. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Antoniou J., Robinson P. J. Structure and expression of genes encoding murine Qa-2 class I antigens. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5920–5924. doi: 10.1073/pnas.82.17.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori T., Tanigaki N., Pressman D. A mouse plasma substance carrying beta2-microglobulin activity and lacking in H-2 alloantigenic activity. J Immunogenet. 1976 Apr;3(2):123–134. doi: 10.1111/j.1744-313x.1976.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ramanathan L., Rogers M. J., Robinson E. A., Hearing V. J., Tanigaki N., Appella E. Biochemical analysis of a 40,000 mol. wt mouse serum protein which binds beta 2-microglobulin and has serological cross-reactivity with H-2 antigens. Mol Immunol. 1982 Sep;19(9):1075–1086. doi: 10.1016/0161-5890(82)90318-2. [DOI] [PubMed] [Google Scholar]
- Sharrow S. O., Flaherty L., Sachs D. H. Serologic cross-reactivity between Class I MHC molecules and an H-2-linked differentiation antigen as detected by monoclonal antibodies. J Exp Med. 1984 Jan 1;159(1):21–40. doi: 10.1084/jem.159.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloski M. J., Uhr J. W., Flaherty L., Vitetta E. S. Qa-2, H-2K, and H-2D alloantigens evolved from a common ancestral gene. J Exp Med. 1981 May 1;153(5):1080–1093. doi: 10.1084/jem.153.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Straus D. S., Stroynowski I., Schiffer S. G., Hood L. Expression of hybrid class I genes of the major histocompatibility complex in mouse L cells. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6245–6249. doi: 10.1073/pnas.82.18.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroynowski I., Forman J., Goodenow R. S., Schiffer S. G., McMillan M., Sharrow S. O., Sachs D. H., Hood L. Expression and T cell recognition of hybrid antigens with amino-terminal domains encoded by Qa-2 region of major histocompatibility complex and carboxyl termini of transplantation antigens. J Exp Med. 1985 May 1;161(5):935–952. doi: 10.1084/jem.161.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Artzt K., Bennett D., Boyse E. A., Jacob F. Structural similarities between a product of the T/t-locus isolated from sperm and teratoma cells, and H-2 antigens isolated from splenocytes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3215–3219. doi: 10.1073/pnas.72.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphisms: most mouse class I genes map to the Tla complex. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Vitetta E. S., Kettman J. R. Cell surface immunoglobulin. XX. Antibody responsiveness of subpopulations of B lymphocytes bearing different isotypes. J Exp Med. 1977 Jun 1;145(6):1421–1435. doi: 10.1084/jem.145.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]