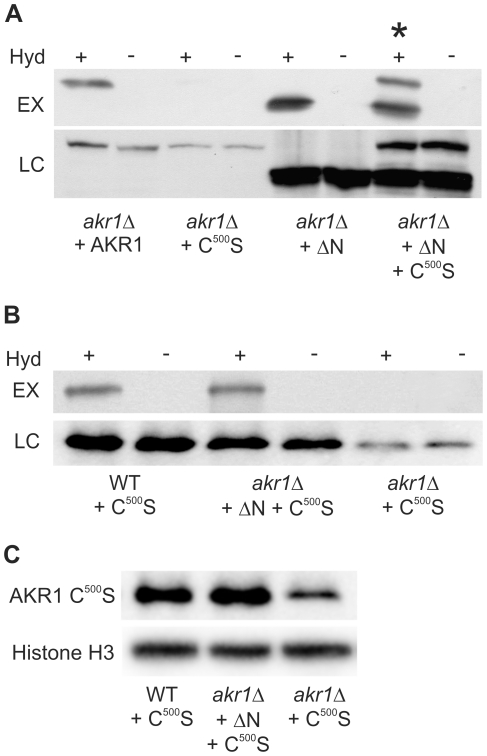
Figure 1. AKR1 S-acylates itself in trans and this does not require the ankyrin repeats.
S-acylation assays of AKR1 variants expressed in yeast were performed using the biotin switch method whereby hydroxylamine is used to specifically cleave S-acyl groups revealing sulfhydryls which are subsequently labelled with biotin. Samples are then passed through a neutravidin column and S-acylation state is assayed as a function of recovery by the column using antibodies against the protein of interest. Negative controls substitute Tris, which does not cleave S-acyl groups, for Hydroxylamine. +indicates presence of hydroxylamine, −indicates absence of hydroxylamine. EX –S-acylated AKR1 variants detected by the biotin switch method, LC – loading control to show the total amount of AKR1 variant (regardless of S-acylation state) in each sample. (A) AKR1 is able to covalently bind acyl groups (auto-S-acylate) and disruption of the DHHC domain by introduction of the C500S mutation abolishes this auto acylation. AKR1 ΔN maintains the ability to auto acylate and co-expression of AKR1 ΔN and AKR1 C500S leads to S-acylation of AKR1 C500S (upper band in lane marked with *). (B) S-acylation of AKR1 C500S in trans occurs in a wild type background. Expression of AKR1 C500S in WT cells (AKR1 fully functional) produces the same S-acylation of AKR1 C500S in trans as co-expression of AKR1 C500S with AKR1 ΔN in akr1Δ cells. (C) Western blot showing that the total amount of AKR1 C500S detected increases in conditions where this protein is S-acylated in trans, suggesting that S-acylation promotes AKR1 stability. Expression of AKR1 C500S in WT cells or co-expression in akr1Δ cells with AKR1 ΔN leads to higher levels of AKR1 C500S being detected than expression of AKR1 C500S alone in akr1Δ cells. LC – loading control: Histone H3.