Figure 3.
Structure of PLA2G5 and Hypothetical Model of Human Group V Phospholipase A2 Binding to a Phospholipid Membrane Surface
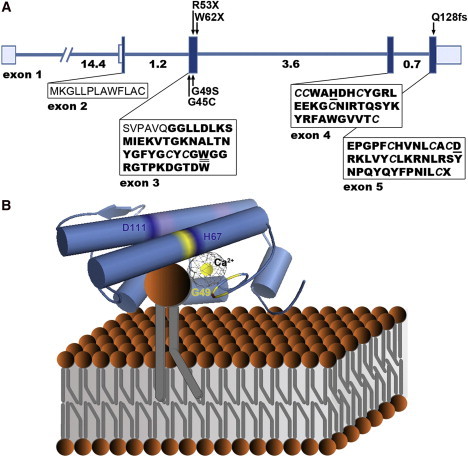
(A) Exons are depicted with boxes in which the shaded areas denote the coding sequence and the unshaded areas denote the 5′ and 3′ untranslated regions. Numbers under the line correspond to intron size (kb), and arrows indicate the position of mutations identified in this study. The amino acid sequence of the signal peptide is shown in normal font; the sequence of the 118 amino acid mature enzyme after cleavage of the prepeptide is shown in bold font (Uniprot8). Cystine residues forming the six disulfide bridges maintaining the enzyme's rigid three-dimensional structure are italicized (Uniprot8). Amino acids responsible for interfacial binding (tryptophan 50)43 and catalytic activity (histidine 67 and aspartic acid 111)12 are underlined.
(B) A homology model of human group V phospholipase A2 (Protein Data Bank accession code 2ghn)44 after hypothetical association with a phospholipid membrane is presented. Structural features of the active site, conserved among secreted phospholipase A2s, are highlighted; these features include a catalytic Ca2+ ion bound by a peptide loop (yellow) and a catalytic dyad formed by amino acids His67 and Asp111 (dark blue).12 The Ca2+ coordination includes carbonyl backbone interactions from Tyr47, Gly49, and Gly51, as well as a shared bidentate interaction from Asp68 (amino acids colored in yellow; Uniprot). Trp50, a key amino acid in the enzyme's interfacial binding surface (distinct from the active site) is highlighted in red; its indole chain contributes to the characteristic ability of group V phospholipase A2 to bind to both zwitterionic and anionic phospholipid vesicles.43 Cationic residues that are also responsible for membrane binding at the carboxyl end of the protein are colored in purple.45
PyMOL (Delano Scientific, Portland, OR) was used for viewing the human group V phospholipase A2 three-dimensional molecular structure (orthoscopic view, cartoon setting, cylindrical helices).
