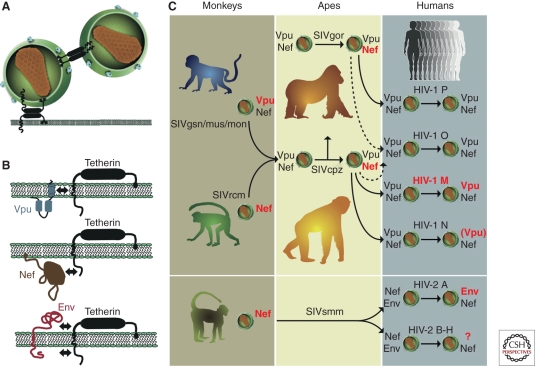
Figure 6.
Tetherin function and virus specific antagonism in different primate hosts. (A) Mechanism of restricted virion release by tetherin; two alternative models are shown (for details, see Evans et al. 2010); (B) Viral antagonists of tetherin and their sites of interaction (indicated by arrows). Vpu associates with the trans-membrane domain of tetherin, Nef targets the cytoplasmic domain, and Env interacts either with the extracellular or the cytoplasmic domain (Kirchhoff 2010; Serra-Moreno et al. 2011). (C) Antitetherin function in HIV-1 and HIV-2 and their immediate simian precursors. SIVcpz acquired vpu and nef genes from different sources, the SIVgsn/mus/mon and SIVrcm lineages, respectively. During adaptation in chimpanzees, Nef (and not Vpu) evolved to become an effective tetherin antagonist. SIVgor and SIVsmm also use Nef to counteract tetherin. After transmission to humans, SIVcpz, SIVgor, and SIVsmm Nef were unable to antagonize human tetherin because of a deletion in its cytoplasmic domain. HIV-1 group M adapted by regaining Vpu-mediated antitetherin activity. The Nef and Vpu proteins of HIV-1 groups O and P remained poor tetherin antagonists. The Vpu of HIV-1 group N gained modest antitetherin activity, but lost the ability to degrade CD4. HIV-2 group A adapted by gaining Env-mediated antitetherin activity; whether HIV-2 groups B–H gained antitetherin function has not been tested. Proteins that are active against tetherin are highlighted in red, and those that are inactive are shown in gray (adapted from Kirchhoff [2010] and reprinted, with permission, from Elsevier ©2010).