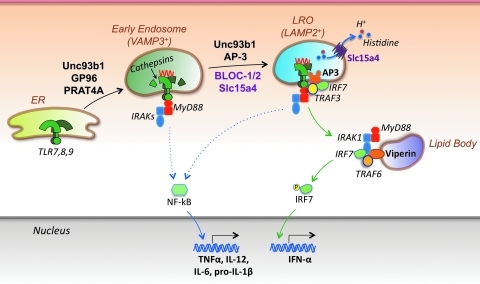
FIG. 2.
TLR compartmentalization and trafficking. Nucleic acid-specific TLRs traffic from the endoplasmic reticulum (ER) to endolysosomes, where they undergo ligand binding and cathepsin-mediated proteolytic cleavage, a process required for efficient signaling. Several proteins have been shown to facilitate TLR translocation to endolysosomes, including the heat-shock protein gp96, PRAT4A, and Unc93b1. Moreover, type I IFN production by pDCs is strictly dependent on TLR localization to specialized compartments termed lysosome-related organelles (LROs). AP-3 (likely together with Unc93b1) mediates TLR translocation to LROs, whereas other molecules in these organelles are required for effective type I IFN induction, including BLOC-1, BLOC-2, and Slc15a4 (a transporter of protons and histidines from the LRO lumen to the cytosol). In addition, the IFN-induced molecule viperin has been shown to localize to lipid bodies and promote assembly of a signaling complex that includes MyD88, IRAK1, and TRAF6, thereby greatly facilitating IRF7 activation and IFN-α production. One study suggests that IRF7 and NF-kB signaling is initiated from LROs and early endosomes, respectively, whereas another study indicates that both pathways are initiated in the LROs.