Abstract
Chronic lung injury resulting from a variety of different causes is frequently associated with the develop ment of pulmonary fibrosis in humans. Although the etiology of pulmonary fibrosis is generally unknown, several sources of evidence support the hypothesis that a number of environmental and occupational agents play an etiologic role in the pathogenesis of this disease. The agents discussed in this review include beryllium, nylon flock, textile printing aerosols, polyvinyl chloride and didecyldimethylammonium chloride. The authors also describe a variety of animal models, including genetically modified mice, in order to investigate the molecular mechanism of pulmonary fibrosis, focusing on chemokine receptors, regulatory T cells and transforming growth factor-β and bone morphogenetic protein signaling. Overall, we propose the concept of toxicological pulmonary fibrosis as a lung disease induced in response to environmental cues.
Keywords: beryllium, nylon flock, DDAC, TGF-β, chemokine receptor, regulatory T cell
Introduction
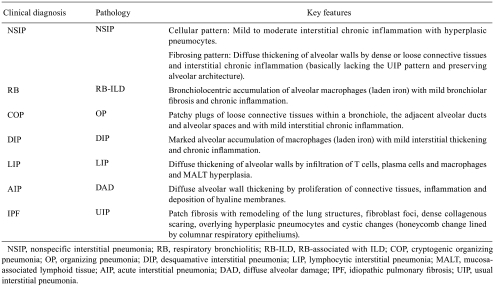
Chronic lung injury resulting from a variety of different causes is frequently associated with the development of pulmonary fibrosis, which is characterized by replacement of the normal alveolar structure by a thickened extracellular matrix (ECM) with consequent reduction in the capacity for gas exchange1. Interstitial lung diseases (ILDs), more accurately known as diffuse parenchymal lung diseases (DPLDs), are a diverse group of pulmonary disorders, which include idiopathic interstitial pneumonias (IIPs), drug-induced ILDs and occupational and environmental lung diseases2. IIPs are categorized as heterogeneous clinicopathologic disorders, which include nonspecific interstitial pneumonia (NSIP), respiratory bronchiolitis (RB)/ RB-associated ILD (RB-ILD), cryptogenic organizing pneumonia (COP)/organizing pneumonia (OP), desquamative interstitial pneumonia (DIP), lymphocytic interstitial pneumonia (LIP), acute interstitial pneumonia (AIP)/diffuse alveolar damage (DAD) and idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP), which have been defined by the American Thoracic Society/European Respiratory Society consensus classification3–5 (Table 1). IPF/UIP is the most common disorder among the IIPs, characterized by unrelenting progression of fibrosis; DIP and RB-ILD are related pathologically; and AIP, characterized by an acute onset of respiratory symptoms, is analogous to acute respiratory distress syndrome, differing only in that it is not preceded by an identifiable catastrophic event5.
Table 1. . Histopathological Pattern of Interstitial lung Diseases (ILD)3–5.
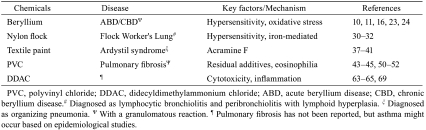
Although the etiology of IPF is generally unknown, several sources of evidence support the hypothesis that a number of environmental and occupational agents such as cigarette smoke, agriculture/farming, livestock, wood dust, metal dust and stone/sand play an etiologic role in the pathogenesis of this disease6. With respect to occupational and environmental lung diseases, the industrial revolution of the 19th century resulted in widespread exposure to dust, which promoted the development of diseases such as silicosis, coal workers’ pneumoconiosis and asbestosis; however, improvement in working conditions resulted in a decrease in the incidence of these lung diseases2. In the past 20–30 years, an increase in industrial processes has led to the production and use of a large number of chemicals, some of which are currently reported as causes of ILDs (e.g., beryllium, nylon flock, textile printing aerosols, polyvinyl chloride (PVC) and organic vapors from flavorings). Sarcoidosis, a distinct entity of ILDs, is a granulomatous disease of unknown etiology, occurring most commonly in the lung; however, noninfectious environmental agents (e.g., beryllium, aluminum and zirconium) can produce pulmonary lesions similar to those of sarcoidosis2. The agents discussed in this review include beryllium, nylon flock, textile printing aerosols, PVC and didecyldimethylammonium chloride (DDAC) (Table 2). Common ILD-causing drugs include amiodarone, antibiotics, nonsteroidal anti-inflammatory drugs, chemotherapeutic agents (e.g., bleomycin and methotrexate) and nitro drugs, all of which can produce virtually all the histopathologic patterns of interstitial pneumonia, including NSIP, OP, DIP and a pulmonary granulomatosis-like reaction7. However, this review does not include drug-induced ILDs, except for a bleomycin animal model, for discussing the mechanism of pulmonary fibrosis.
Table 2. Environmental and Occupational Agents and Pulmonary Fibrosis.
The pathogenesis of pulmonary fibrosis is complex, involving innate and adaptive immunoreactions against exogenous environmental cues and subsequent molecular and cellular responses, leading to an imbalance of profibrogenic and antifibrogenic effects in healing processes. This review also focuses on specific pathobiological mediators such as chemokines and chemokine receptors, regulatory T cells (Tregs) and transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) signaling.
Beryllium
Beryllium, which mostly occurs naturally as beryllium aluminum silicate, is the fourth lightest element, having a low density (1.85 g/cm3 at 20 °C), high melting point (1287 °C) and high tensile strength, and these properties have led to its incorporation into many high-technology applications (e.g., electronics, aerospace, machinery and nuclear weapons manufacture)8,9. Exposure to beryllium at workplaces continues to be a public health concern, with approximately one million individuals exposed and potentially at risk of developing acute beryllium disease (ABD) and chronic beryllium disease (CBD), both of which involve hypersensitivity reactions to beryllium10,11. Genetic susceptibility to CBD has been linked to a major histocompatibility complex (MHC) class II isotype, human leukocyte antigen (HLA)-DP alleles, which contain a glutamic acid residue at amino acid position 69 in the β-chain12–14. Among the mouse strains FVB/N, AKR, Balb/c, C3H/HeJ, C57/BL6, DBA/2 and SJL/J, the FVB/N strain has been reported to be the least responsive, while the SJL/J and C57/BL6 strains have been shown to be the highest responders15.
CBD is characterized by the presence of noncapsulating granulomatous inflammation in the lung and subsequent development of progressive fibrosis16. Accumulating evidence clearly suggests that CBD is recognized as an immune-mediated granulomatous reaction. Six to nine months after a single exposure of A/J and C3H/HeJ mice to beryllium for 90 min, lung responses comprised interstitial aggregates of lymphocytes—B cells centrally and T cells peripherally—and granulomatous pneumonia characterized by intra-alveolar foamy macrophages, giant cells and neutrophils, and multifocal interstitial granulomas17. Evidence suggests that recruitment of beryllium-specific CD4+ T cells to the lung significantly mediates the immunopathogenesis of CBD. These CD4+ T cells expressed an effector memory T cell phenotype10,13 and secreted the T helper 1 (Th1)-type cytokines interferon-γ (IFN-γ), interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α) upon beryllium exposure18–20. Programmed death-1, which is a member of the CD28 family and a negative regulator of T cell function, was expressed at significantly high levels in bronchoalveolar lavage (BAL) CD4+ T cells from CBD patients, plausibly leading to induction of T cells and preventing activation-induced cell death21. Lately, ABD has also been recognized as a specific lymphocytic alveolitis rather than a nonspecific inflammatory process of an irritant11. Beryllium-specific CD8+ T cells may play a minor role, if any, in the pathogenesis of this disease, as inferred from the results of analysis of BAL samples from CBD patients10.
Beryllium-induced oxidative stress plays a role in the pathogenesis of granulomatous inflammation in CBD. A decrease in intracellular thiols, glutathione and cysteine was observed in peripheral blood mononuclear cells from beryllium-sensitized and CBD patients, and beryllium-mediated T cell proliferation was inhibited by the thiol antioxidant N-acetylcysteine and the catalytic antioxidant manganese (III) in vitro22. Increased glutathione in lung epithelial lining fluid from CBD patients is correlated with glutathione peroxidase activity23. Heme oxygenase-1 (HO-1) activity is also increased in induced sputum from CBD patients24. Together, the data imply that augmentation of glutathione- and HO-1-mediated antioxidant systems may be involved in the process of CBD.
Although cellular and molecular mechanisms underlying berylliosis have been investigated, the exact profibrogenic factors have not been identified thus far. Mast cells may contribute to the pathogenesis of CBD, serving as an important source of a 17.8-kDa isoform of basic fibroblast growth factor, which is a potent mitogenic and chemotactic factor, regulating fibroblast proliferation and ECM production25. In contrast, beryllium at low concentrations inhibits the growth of normal human fibroblasts, as proved by G0G1/pre-S phase arrest and senescence associated with elevation of the p53, p21 and p16 levels26,27. At high concentrations, beryllium is somewhat carcinogenic to BALB/c-3T3 cells (mouse embryonic fibroblast cell line)28. TGF-β signaling in CBD has not been fully investigated, although Jonth.et al. (2007) have reported that the codons- 509 C and 10T, implicated in the production of low levels of TGF-β1 protein, are shared susceptibility factors associated with more severe granulomatous inflammation in CBD29. Thus, investigations of profibrogenic/antifibrogenic signaling would provide a better understanding of the pathobiology of CBD and aid in exploring therapeutic targets.
Nylon Flock and Textile Paints
Flock is cut or pulverized fiber—synthetic or natural—of small diameter that produces a velvet-like coating when applied to an adhesive-coated fabric or other material. Flocked fabrics of nylon (polyamide), rayon (cellulose acetate), Dacron (ethylene glycol-terephthalic acid) and polyester fibers are becoming increasingly popular, especially as upholstery coverings and blankets.Kern et al. (2000) reported a chronic nongranulomatous ILD termed “Flock Worker’s Lung” in nylon flocking industry workers at a Rhode Island plant in the 1990s30. The workers developed BAL eosinophilia, NSIP, OP and/or lymphocytic bronchiolitis and interstitial fibrosis. A small number of North American patients have also been reported to have nylon flock-related DIP. At a clinical pathology workshop hosted by the National Institute for Occupational Safety and Health, consulting pulmonary pathologists diagnosed “lymphocytic bronchiolitis and peribronchiolitis with lymphoid hyperplasia” in workers at 5 nylon flock facilities in 3 different U.S. states and a Canadian province, on the basis of clinical examination and histopathological findings31. This descriptive terminology was supported by the Centers for Disease Control (CDC) at a clinical-pathological workshop hosted by the CDC32.
Although nylon fibers may act as haptens and trigger an allergic reaction (e.g., occupational asthma)33, in vivo experiments have failed to demonstrate pulmonary hypersensitivity reactions to nylon. Airborne dust (nylon flock) collected at a nylon flocking plant interacted with alveolar macro-phages and induced mild-to-moderate, multifocal, suppurative pneumonia around bronchioles after intratracheal instillation in rats (10 mg/kg body weight)34. In rats exposed to aerosols of uncoated, finish-free nylon respirable-sized, fiber-shaped particulates at 6 hours per day and 5 days per week for 4 weeks, there were no significant increases in lung weight, indications of pulmonary inflammation or alveolar macrophage functional deficits, as compared with the control rats35. Thus, since workers exposed to high concentrations of nylon fiber dust may be at an increased risk of ILDs, suitable animal models should be developed to clarify the role of nylon flock in immune-based pulmonary diseases.
Synthetic fibers do not include metals. Synthetic fibers present an insoluble solid-liquid interface to the surrounding host tissue, and the fiber surface includes functional groups having the ability to mobilize metal from endogenous sources, introducing a metal-catalyzed oxidative stress in the lower respiratory tract. Ghio et al.(2006) reported that a ferruginous body, with its accumulated iron, functions as an indicator of both metal accumulation and as a potential oxidative stressor36. The clinical presentations of 2 patients employed in a textile mill were consistent with a chronic fibrotic disease of the lung, in which there were areas of traction bronchiectasis with chronic inflammation and bronchial metaplasia and bronchiolitis with mixed lymphocytic and eosinophilic infiltration. Staining of resected tissue for iron revealed structures having the appearance of ferruginous bodies. Energy dispersive X-ray analysis and scanning electron microscopy revealed that the fibers isolated from the lung were carbonaceous and originated from the mill. Thus, metal, particularly iron, may play a role in the pathogenesis of Flock Worker’s Lung.
Besides synthetic fibers, textile paints may affect human health in these industries. An outbreak of severe respiratory disease called “Ardystil syndrome” occurred in the Community of Valencia, Spain, among factory workers who worked where textiles were air-sprayed with dyes by using the Acramin F paint system. Twenty-two cases of OP were identified, and of the 22 patients, 6 died within a few months37. Besides OP, interstitial fibrosis and DAD were evident38. In another study, after a 1 year follow-up of 27 patients heavily exposed to textile sprays, symptoms were observed to persist in 15 cases39. The main polycationic components of textile sprays are Acramin FWR (a poly-urea), Acramin FWN (a polyamide-amine) and Acrafix FHN (a polyamine), and the non-polycationic component is Acramoll W. Acramin FWR, Acramin FWN and Acrafix FHN exhibited considerable toxicity in rat and human type II pneumocytes and alveolar macrophages, while Acramoll W was almost nontoxic40. Consistent with the results of in vitro experiments, lung damage was caused by Acramin FWR, Acramin FWN, Acrafix FHN or their mixture after intratracheal instillation in hamsters41. Protein concentration, lactate dehydrogenase (LDH) activity, inflammatory cell number and percentage of polymorphonuclear neutrophils were increased in BAL fluid during the first week, and lung weight remained high for at least a month after intratracheal instillation. Inflammatory cell infiltration and subsequent fibrosis with collagen deposition were detected in lung histology, which was confirmed by increased hydroxyproline content in dried lung tissue. Acramoll W did not show toxic effects even in vivo. Interestingly, multiple positive charges might play an important role in the toxicity mechanism of the polycations with no irritant properties, since the cytotoxicities decreased in the presence of polyanions42. Overall, combined exposures to textile dyes and nylon flock could be involved in development of the diseases, and cross-talk between them should be further investigated.
PVC
PVC is the second most commonly used plastic material worldwide, and it is used in numerous products, e.g., credit cards, computer keyboards, flooring material, food containers, medical cannulae, telephones, highway sound barriers and toys. At the end of the synthesis process, the material may exist as respirable dust, the inhalation of which may result in lung function impairment. Epidemiological surveys have identified PVC dust as an etiologic agent in dyspnea with decreased pulmonary function and appearance of opacities in chest radiograph43, in a particular type of pulmonary fibrosis with a granulomatous reaction44 and in interstitial fibrosing pneumonia45. Electron microscopy revealed a nonhomogenous material in the cytoplasm of giant multinucleated cells and/or alveolar macrophages, which was identified to be PVC particles44,46,47. Human alveolar macrophages that engulfed PVC powder showed a similar ultrastructural appearance46. It is important to note that early PVC pneumoconiosis may regress in human cases48.
Several studies on PVC exposure to animals have been conducted, although there is no evidence of fibroproliferation in the lung. Rats, guinea pigs and monkeys were exposed by inhalation (6 hours per day, 5 days per week) for 22 months; autopsies of rats and guinea pigs and of monkeys were performed after 12 and 22 months of exposure, respectively. PVC particles were found in aggregated alveolar macrophages, but there were no other effects either on lung histology or pulmonary function49. Hoet and his colleagues have conducted detailed investigations of the effects of PVC exposure in vitro and in vivo. Emulsion of PVC particles with a mean diameter of 2 µm exhibited moderate toxicity in cultures of different pulmonary cells, i.e., rat and human alveolar macrophages, rat type II pneumocytes, A549 cells (type II cell type) and THP-1 cells (macrophage-like). It was found that the cytotoxicity and inflammatory potential of some PVC particles might be mostly due to their residual additives, since removal of the residual additives considerably reduced their toxic effects50. However, in vivo experiments did not confirm the conclusion from the in vitro toxicity tests. Male Wistar rats received a single intratracheal instillation of the washed “additive-free” PVC particles. As a result, they exhibited pulmonary inflammation and damage similar to that in the silica-exposed rats at 2 days; however, at 90 days, most parameters returned to the control level, except for minor histopathological lesions. Interestingly, additive-free PVC particles caused less neutrophil but more eosinophil influx than the original PVC particles containing residual detergents. The washed additive-free PVC particles might be more hydrophobic than the original PVC particles, but pulmonary eosinophilia was induced via an unknown mechanism51. Repeated intratracheal instillation of PVC particles for 3 weeks increased the number of CD4+ and CD8+ T cells in BAL fluid, and compared with the original PVC particles, the additive-free PVC particles tended to induce more changes in many endpoints, including BAL eosinophilia52, as observed in the single intratracheal instillation study51. Experimental BAL eosinophilia is apparently linked to asthma, as reported in epidemiological studies on PVC53,54. Together, deposition of PVC particles with or without residual detergents in alveolar macrophages and subsequent immunoreactions may contribute to the development of PVC-induced pneumoconiosis.
DDAC
DDAC [C10H21N(CH3)2C10H21·Cl], a representative dialkyl quaternary ammonium compound (QAC), is used as a detergent in wood preservatives, as a disinfectant against pathogens and in other applications55–58. DDAC formulations are directly added to water in swimming pools, spas and humidifiers and used for treatment of surfaces in institutional, commercial, industrial and residential settings by fogging, flooding, immersion, wiping, mopping, aerosol spray treatment and low- and high-pressure spray treatments with final spray concentrations ranging from 0.5 to 26320 ppm59. The action of DDAC on the cell membrane causes leakage of intracellular molecules60, together with autolysis and subsequent death of Escherichia coli and Staphylococcus aureus61. Occupational exposure to QACs, including DDAC, therefore, is known to cause contact dermatitis, conjunctivitis62 and asthma63–65 among professionals working in healthcare and cleaning industries. Although DDAC does not seem to contaminate the indoor hospital atmosphere during the disinfection process, it can contaminate working atmospheres if it is put in suspension by aerosolization66. The same health concerns are expected to exist for veterinary professionals, since DDAC is a useful disinfectant for preventing the entry and spread of infectious disease agents, including enveloped and non-enveloped viruses, in domestic animals67,68.
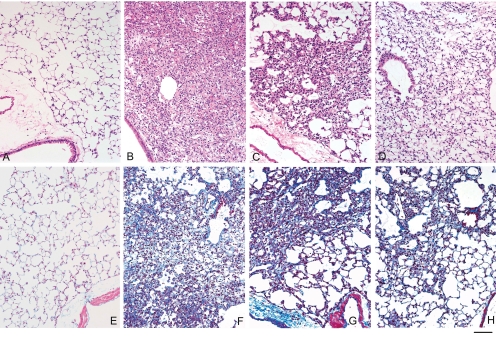
Recently, we reported that DDAC induces pulmonary injury and fibrosis in mice69. To our knowledge, this was the first report on pulmonary fibroproliferative response to topically applied DDAC. In mice with intratracheally instilled DDAC, pulmonary cytotoxicity (increase in protein concentration and LDH activity in BAL fluid) was evident in association with inflammation, which was confirmed by the expression of monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP-1β and regulated upon activation, normal T cell expressed and secreted (RANTES) in BAL fluid. These changes were accompanied by altered gene expression of the chemokine (C–C motif) receptors Ccr1, Ccr2, Ccr3 and Ccr5 in the lung. Expression of chemokines might principally be derived from alveolar macrophages, as in vitro cytotoxicity was higher in the macrophages (J774.1) than in epithelial cells (A549) and isolated mouse lung fibroblast (MLF)-like cells (Ohnuma et al., unpublished data). The cytotoxic and inflammatory phases were accompanied or followed by pulmonary remodeling, i.e., fibrosis, which was evident by an increase in interstitial connective tissue and fibroblasts/ myofibroblasts, as demonstrated by Masson’s trichrome stain; immunohistochemistry for α-smooth muscle actin (α-SMA); and mRNA expression of type I procollagen69. Overall, pulmonary injury peaked in the first week after a single instillation of DDAC in the lung; fibroproliferation gradually became profound in the first week and peaked in the second week, subsequently reducing in the third week (Fig 1). The role of TGF-β and BMP in fibrogenesis is currently being investigated in our laboratories.
Fig. 1.
DDAC induces inflammation and fibroproliferation in the lungs. Mice were intratracheally instilled with 0.01% of DDAC or the vehicle control and were sacrificed on day 7, 13, or 20 after the treatment69. Lung tissue samples were subjected to histopathological examination. Representative images of the lung tissues stained with hematoxylin and eosin (A–D) and Masson trichrome (E–H) from the vehicle control (A, E) and DDAC-treated mice on day 7 (B,F), 13 (C,G) or 20 (D,H). Scale bar: 50 µm.
Limited toxicology information is currently available for DDAC59,70. Similar to DDAC, benzalkonium chloride (BAC) is a mixture of alkylbenzyldimethylammonium chlorides belonging to the QAC group and is widely used as a disinfectant and in preservatives and stabilizers. Rats that aspirated BAC following oral administration showed AIP71. Furthermore, BAC inhalation caused strong inflammation via an irritant activity, as demonstrated by an increase in lung weight and high levels of total protein, LDH activity, matrix metalloproteinase-9 (MMP-9), TNF-α and MIP-2 in BAL fluid; however, no histopathological examinations were carried out72. The mode of action of DDAC and BAC hypothetically poses human health concerns, particularly if people are unknowingly exposed to these QACs or experience prolonged workplace exposure. In our current protocol, a similar sequence of pulmonary changes as those occurring in bleomycin- or fluorescein isothiocyanate (FITC)-induced lung injury73–77 was observed on DDAC administration; it seemed that the fibrotic response to DDAC administration was more rapid, but weaker, than that to bleomycin or FITC treatment. This unique property of pulmonary responses would be helpful in better understanding pulmonary fibrotic diseases particularly at promoting and regenerating phases.
Molecular Targets Involved in the Pathogenesisof Pulmonary Fibrosis
Chemokines and CCRs
Inflammatory reactions, involving an influx of particular cell types expressing cytokines, chemokines and cell surface molecules, precede and accompany pulmonary fibrosis, whereas nonspecific inflammatory processes end with nonfibrous tissue repair78. Several cytokines and chemokines and their receptors have been investigated with regard to their contribution to the pathogenesis of pulmonary fibrosis in human and animal models75,79–82.
Murine MCP-1, also known as JE or chemokine (C–C motif) ligand 2 (CCL2), is considered homologous to human CCL2, which is produced by a variety of cells, including endothelial cells, fibroblasts, monocytes, and smooth muscle cells83. MCP-1 and its receptor CCR2 are involved in fibrosis via regulation of profibrotic cytokine generation and ECM formation84–86. This is supported by the fact that CCR2knockout mice with FITC- or bleomycin-induced pulmonary fibrosis showed improvement in fibrosis74,87. The protective effect was associated with suppressed macrophage infiltration and macrophage-derived MMP production88 and enhanced alveolar epithelial cell inhibition of fibroblast proliferation89. CCR2 also mediates neutrophil recruitment through effects on intravascular adherence and subsequent transmigration90. Surprisingly, MCP-1 binds not only to alveolar macrophages but also to bone marrow-derived fibrocytes, both of which express CCR291. Interestingly, transplant of CCR2+/+ bone marrow cells into CCR2–/– recipients restored recruitment of lung fibrocytes and susceptibility to FITC-induced fibrosis. The study showed that fibrocytes may contribute to fibrogenesis in several ways: (1) fibrocytes may directly contribute to fibrosis by secreting collagen, since the amount of collagen secreted by CCR2+/+ fibrocytes exposed to MCP-1 was greater than that secreted by CCR2–/– fibrocytes; (2) fibrocytes secrete TGF-β1, and thus, CCR2-mediated recruitment of fibrocytes to the lung may serve to activate resident fibroblasts via the secretion of TGF-β1; and (3) fibrocytes may differentiate into effector fibroblasts. Murine MCP-5/CCL12, which is homologous to human CCL2, may have a higher affinity for activated macrophages than MCP-192. Moore et al. (2006) proposed that MCP-5 rather than MCP-1 is more likely to drive fibroproliferation in mice via its interaction with CCR293. Inconsistent with this, Tsou et al. (2007) demonstrated that MCP-1 and MCP-3, rather than MCP-2 and MCP-5, are the CCR2 agonists that are most critical for the maintenance of monocytes94. Thus, although the contribution of CCR2 agonists remains controversial, the MCP/CCR2 system may represent the link between the macrophage-dominant phase and the subsequent remodeling phase.
CCR1 is constitutively expressed on monocytes, neutrophils, lymphocytes and eosinophils and interacts with MIP-1α, RANTES, MCP-2 and MCP-383,95. After bleomycin challenge in mice, CCR1 mRNA levels and CCR1-positive cells increased and peaked on day 7, which paralleled the expression of MIP-1α and RANTES73. Anti-CCR1 antibody inhibited inflammatory cell accumulation and collagen deposition, resulting in dramatic improvement in survival. In contrast, bleomycin-induced an increase in intrapulmonary macrophage and fibrocyte numbers, and collagen deposition was attenuated in MIP-1α–/– mice but not in CCR1–/– mice77. CCR1 was observed to play a limited role in pulmonary granuloma formation, since the granuloma size remained unchanged in CCR1–/– mice, as compared with CCR1+/+ mice, challenged with Sepharose beads coupled to the purified protein derivative of Mycobacterium bovis or soluble antigens derived from Schistosoma mansoni eggs96. However, natural killer (NK) cell recruitment and Th1 response (expression of IL-2 and IFN-γ) were reduced, while Th2 response (expression of IL-5 and IL-13) was enhanced in CCR1–/– mice in comparison with CCR1+/+ mice. Thus, CCR1 seemed to be critical in immune-mediated fibrotic disease, and this was supported by the fact that airway remodeling, e.g., goblet cell production and subepithelial fibrosis, was absent in CCR1–/– mice during chronic fungal allergic airway disease97. Rather than CCR1, CCR5, another receptor of MIP-1α, seemed to be critical in regulating fibrosis, since CCR5–/– mice showed attenuation of collagen deposition and macrophage infiltration induced by bleomycin77.
CCR3 is highly expressed on neutrophils and eosinophils, and its ligand is eotaxin-1/CCL1183,95. Administration of bleomycin induces a marked pulmonary expression of eotaxin-1 and CCR376. Eotaxin-1–/– mice showed reduced pulmonary fibrosis associated with a decrease in TGF-β1 mRNA expression, whereas increased expression of eotaxin-1 by using eotaxin-1-expressing adenovirus enhanced bleomycin-induced lung fibrosis. Similar to eotaxin-1–/– mice, those treated with neutralizing CCR3 antibodies showed reduced pulmonary fibrosis, eosinophilia, neutrophilia and TGF-β1 mRNA expression.
CCR4 is a selective marker for Th2 lymphocytes and is expressed in dendritic cells, NK cells and monocytes; thymus- and activation-regulated chemokine (TARC)/ CCL17 and macrophage-derived chemokine (MDC)/CCL22 are both high-affinity ligands and high-potency agonists for CCR483,95,98. The mRNA and protein expressions of TARC and MDC were elevated in lung tissues after bleomycin treatment, which was related to alveolar expression of CCR499. Neutralization of TARC, but not MDC, led to a reduction in collagen deposition and BAL leukocytes, including CD4+ T cells, CD8+ T cells, NK cells, macrophages and neutrophils. TARC was expressed in alveolar epithelial cells in the lung tissues of bleomycin-treated mice and human patients with IPF. These data support the results of an experiment conducted using CCR4–/– mice, which protected from bleomycin-induced lethal inflammatory and fibrotic responses, with evidence of a reduction in the TARC and MDC levels observed in lung homogenates100. A major contribution to this protective effect might be the regulatory role of CCR4 in determining the phenotype of macrophages during the acute inflammatory phase by switching classically activated or M1 macrophages (type-1 polarization, induced by Th1 signals, i.e., IFN-γ expression) to alternatively activated or M2 macrophages (type-2 polarization, induced by Th2 signals, i.e., IL-4 and IL-13 expression). In the presence of CCR4, TARC and MDC promoted tissue injury through induction of the M1 macrophage phenotype with increased nitric oxide synthase 2 (NOS2) and decreased arginase 1 expression; in the absence of CCR4, M2 macrophage activation occurred through suppression of NOS2 and increased expression of arginase 1 and found in inflammatory zone 1 (FIZZ1), favoring upregulation of a decoy receptor, D6, which attenuates inflammation and tissue injury by internalizing and degrading chemokines. Thus, it appears that alterations in M1/M2 marker expression are confined to the early inflammatory stage and that the establishment of a fibrotic process is not necessarily associated with M2 polarization101. Furthermore, reduction in arginase 1 in lung epithelial cells enhanced the levels of NO metabolites and S-nitrosylated proteins and resulted in increased TNF-α- or lipopolysaccharide (LPS)-stimulated nuclear factor-κB (NFκB) DNA binding102. Coculture of FIZZ1-expressing type II alveolar epithelial cells with fibroblasts stimulates α-SMA and type I collagen expression independent of TGF-β103 and dependent on Notch/Jagged 1104. The imbalance of arginase 1 and NOS2 in association with the induction of FIZZ1 may contribute to fibrogenesis, depending on the stage of inflammation and specific cell types.
CCR7 is expressed by T and B cells and plays an important role in T cell and dendritic cell trafficking in association with its ligands, Epstein-Barr-induced 1 (EBI1)-ligand chemokine (ELC)/CCL19 and secondary lymphoid-tissue chemokine (SLC)/CCL2183,98. CCR7 expression is raised in a severe form of IIP, UIP, and localized to focal areas consisting of activated resident fibroblasts, rather than myofibroblasts and bone marrow-derived fibrocytes105. This was supported by Pierce et al. (2007), who showed that CCR7 is expressed by primary IPF/UIP fibroblasts but not by normal fibroblasts. IPF/UIP fibroblasts showed migration and proliferation in response to CCR7 activation by SLC, but not by ELC, and SLC-mediated CCR7 activation seemed to be limited to the extracellular signal-regulated kinase (ERK)1/2 and 90-kDa ribosomal S6 kinase (p90RSK) pathways, since the p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways were not activated by SLC106. The investigators adopted an unusual technique to clarify the role of SLC/CCR7 axis elegantly. IPF/UIP fibroblasts, NSIP fibroblasts and normal fibroblasts were intravenously injected into severe combined immunodeficiency (SCID) mice lacking adaptive and innate immune features. Interestingly, the mice with IPF/UIP fibroblasts showed patchy interstitial fibrosis and those with NSIP fibroblasts showed a more diffuse interstitial fibrosis, with an increased hydroxyproline content in both cases. The injected normal fibroblasts did not induce interstitial remodeling. Neutralization of SLC or CCR7 attenuated pulmonary fibrosis in mice that received either type of fibroblasts. However, delayed onset of pulmonary remodeling, which was noted at 35 days after intravenous injection, remains to be clarified. Similarly,Trujillo et al. (2010) showed that CCR7–/– mice were protected from bleomycin-induced pulmonary fibrosis; the effect was related to an early increase in lung CD4+ T cells and late increase in CD4+CD25+FoxP3+ Tregs, which was consistent with increased IL-2 expression107, indicating that CCR7-independent Tregs expansion is a novel therapeutic target for pulmonary fibrosis.
Tregs
T cells are present in the lungs of patients with pulmonary fibrosis because a variety of causes play a pivotal role in the fibrotic process. CD4+ T cells are abundant in the lung in granulomatous lung diseases such as IPF, sarcoidosis and berylliosis10,13,108. In fact, CD4+ T cells from IPF patients have characteristics typical of cell-mediated pathologic responses, including augmented effector functions (production of TGF-β1, IL-10 and TNF-α), provision of facultative help for immunoglobulin G (IgG) autoantibody production, oligoclonal expansions and proliferations driven by an antigen present in diseased tissues109. Most previous studies investigated whole T cells, while the emerging novel sub- populations of naturally occurring and adaptive Tregs take the center stage as the crucial immunoregulatory cells that are capable of suppressing Th1- and Th2-mediated adaptive immune responses in a cell contact-dependent fashion directly or by acting on antigen-presenting cells110. Since the transcription factor Foxp3 has been shown to be selectively expressed in Tregs in humans and mice, Foxp3 mRNA represents a more specific marker than cell surface molecules such as CD25, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and glucocorticoid-induced TNF receptor (GITR)111–114.
In CBD and beryllium-sensitized patients, a small population of BAL CD4+ T cells retained CD27 expression, and these CD4+CD27+ T cells contained the FoxP3-expressing naturally occurring Treg subset, increasing T cell expansion, while the vast majority of CD4+ T cells had lost surface CD27 expression, which inversely correlated with lung inflammation in the patients115. The number of CD4+CD25+FOXP3+ Tregs and their activities (demonstrated by Th1- and Th2-cytokine expression) were reduced in both BAL and peripheral blood of patients with IPF as compared with those of healthy volunteers and patients without IPF; further, the defective function of BAL Tregs highly correlated with the parameters of disease severity, as determined by pulmonary function tests116. Furthermore, downregulation of CD28, which induces FoxP3 and generates CD4+CD25+ Tregs, was evident in IPF patients, and these patients demonstrated infiltrations of CD4+CD28null T cells in lung tissues and a positive correlation between the presence of these cells and indication of lung transplantation or death in the next year117. Thus, the data suggested that Tregs play a pivotal role in pulmonary fibrosis; however, additional studies extensively addressing Treg interactions with cellular and soluble factors in animal models and human patients are required, since it has been reported that Treg depletion accelerates in vitro granuloma growth in mononuclear cell cultures of healthy controls but not in those of patients with active sarcoidosis118.
TGF-β and BMP signaling
TGF-β1 has the ability to induce the expression of ECM proteins in mesenchymal cells119. Cellular TGF-β1 mRNA levels were higher than cellular TGF-β2 and TGF-β3 mRNA levels in IPF patients120 and bleomycin-treated mice121. However, TGF-β2 and TGF-β3 have the potential for in vitro fibrogenic activity that is the same as or higher than the in vitro fibrogenic activity of TGF-β1122. Signaling by TGF-β family members occurs via TGF-β type I and type II receptors (TGF-βRI and TGF-βRII, respectively)119,123,124. Upon phosphorylation by activated TGF-βRI/activin receptor-like kinase-5 (ALK-5), TGF-β-activated Smad2/Smad3 forms a heteromeric complex with Smad4, which is trans-located to the nucleus where it functions as a transcription factor. Smad3, in particular, is of critical importance in the development of fibrosis124,125, since loss of Smad3 greatly attenuated histopathological fibrotic responses to bleomycin126 or active TGF-β1-expressing adenovirus127 in the mouse lung. Active TGF-β1 is a major cytokine that stimulates transcription of the Col1α2 gene, as determined by the binding of Smad3-Smad4 complex to the cis-element TGF-β-responsive element (TbRE) in the proximal promoter region128.
It is important to note that repression of collagen gene expression is critical for pulmonary disease therapy, and lately, two complex underlying mechanisms have been proposed by Xu et al. (2006, 2007): methylation-mediated repression may be mediated by interaction of the regulatory factor for X-box 1 (RFX1) and histone deacetylase 1 (HDAC1) at the TATA box, or IFN-γ-mediated repression may be mediated by the interaction of RFX5, class II trans-activator, HDAC2, and peroxisome proliferator-activated receptor γ at the CCAAT and TATTA boxes129,130. Lin et al. (2006) identified protein phosphatase 1A (PP1A)/PP2Cα as a Smad2/Smad3 SXS motif-specific phosphatase that inhibits Smad2/Smad3 phosphorylation131. Interestingly, phosphatase and tensin homolog (PTEN) deleted on chromosome 10 plays a negative role in the TGF-β pathway by forming a complex with PP1A/PP2Cα132. A lipid mediator, sphingosine 1-phosphate (S1P), mimics TGF-β-induced cell responses through cross-activation of Smad signaling133 or Rho signaling134 cascades, while dihydrosphingosine 1-phosphate (dS1P) plays an opposite role in the regulation of TGF-β signaling by stimulating phosphorylation of PTEN and its subsequent translocation to the nucleus132. Thus, repression of collagen gene expression and negative regulation of TGF-β/Smad signaling through formation of a PP1A/PP2Cα-PTEN complex, probably mediated by dS1P,may open the possibilities to control the progression of pulmonary fibrosis.
Further, TGF-β terminates the induction of its own target genes by induction of Smad7, which competes with Smad2 and Smad3 for binding to TGF-βRI125; inhibits receptor-activated Smad2 phosphorylation135; recruits the E3 ubiquitin ligases Smurf1, Smurf2, Nedd4-2 and WW domain-containing protein 1 (WWP1)/Tiul1 to TGF-βRI and Smads, causing their degradation136; or specifically binds to the Smad-responsive element via its Mad homology 2 (MH2) domain and disrupts formation of a TGF-β-induced functional Smad-DNA complex137. As expected, a decrease in the expression of Smad7 was observed after bleomycin instillation in rats, in contrast to a persistent increase in the phosphorylation and nuclear localization of Smad2/Smad3138. The same groups compared the levels of phosphorylated Smad3 and Smad7 in isolated fibroblasts from the lungs of control and bleomycin-treated rats under normal conditions and after TGF-β treatment139. The basal levels of phosphorylated Smad3 were higher in the normal lung fibroblasts than in bleomycin-treated lung fibroblasts, but the basal levels of Smad7 were comparable. After TGF-β treatment, the levels of phosphorylated Smad3 and Smad7 mRNA were increased in both treated and normal cells, while the protein level of Smad7 was increased in the normal lung fibroblasts as compared with the bleomycintreated lung fibroblasts. The investigators concluded that inadequate response to TGF-β and consequent imbalance between the levels of Smad7 and phosphorylated Smad3 might contribute to the fibrotic phenotype characteristic of activated fibroblasts. In fact, bleomycin-induced collagen deposition and phosphorylation of Smad3 were prevented by an intratracheal injection of a recombinant adenovirus carrying mouse Smad7 cDNA140. Intriguingly, inhibition of TGF-β2 release by IFN-γ141 and myofibroblast differentiation by hepatocyte growth factor (HGF)142 has been reported to be dependent on Smad7. Overall, overexpression of Smad7 may be a therapeutic target for IIPs.
BMPs member, one of the TGF-β family, have been investigated in pulmonary vascular disorders, especially pulmonary hypertension, which has been linked to mutations in the BMP receptor II gene123 and expression of BMP-2 and BMP-4. BMP-2 expression is increased by proinflammatory stimuli and induces endothelial dysfunction, oxidative stress, and endothelial activation, including increased adhesion of a monocyte cell line143. BMP-4 promotes migration of vascular smooth muscle cells, possibly contributing to vascular remodeling, i.e., medial wall thickening and muscularization of vessel walls144. Lately, the role of BMPs, particularly BMP-7 and BMP-4, and that of gremlin in pulmonary fibrosis has been investigated. BMP-7 is known to act as an antagonist of TGF-β1-dependent fibrogenic activity in mouse pulmonary myofibroblasts by inducing inhibitor of differentiation 2145, which is an important myoepithelial cell marker during epithelial-mesenchymal transition146,147. However, the role of BMP-7 in fibrosis remains controversial, since BMP-7 treatment significantly reduced the hydroxyproline content in asbestos-treated mice148 but not in bleomycin-treated mice149. No alterations in BMP-4 levels have been reported in lung samples of IPF patients, but IPF fibroblasts are less responsive to exogenous BMP-4, which inhibits the growth of and induces apoptosis in myofibroblasts120. However, BMP-4 and BMP-7 accurately regulate fibrogenesis in different ways: BMP-4, but not BMP-7, reduces TGF-β1-induced ECM production, while BMP-7, but not BMP-4, inhibits TGF-β1-induced myofibroblast transformation in normal human lung fibroblasts150. On the other hand, TGF-β1 induces the expression of a BMP inhibitor, gremlin, in A549 lung epithelial cells, and IPF fibroblasts and their lung samples show high levels of gremlin mRNA and protein120,148. Pulmonary gremlin mRNA levels were also upregulated in asbestos-treated mice148. Increased gremlin level may decrease BMP-4-mediated myofibroblast apoptosis and increase TGF-β signaling through suppression of BMP signaling. This concept was supported by evidence of latent TGF-β-binding protein-4–/– fibroblasts, which exhibited reduced activation of TGF-β due to increased expression of BMP-4 and decreased expression of gremlin151. Thus, profibrotic and antifibrotic signaling regulated by TGF-β and BMPs and the role of gremlin in disrupting the balance between both the mediators provides the complex feedback loop coordinating fibrogenesis152.
Conclusion
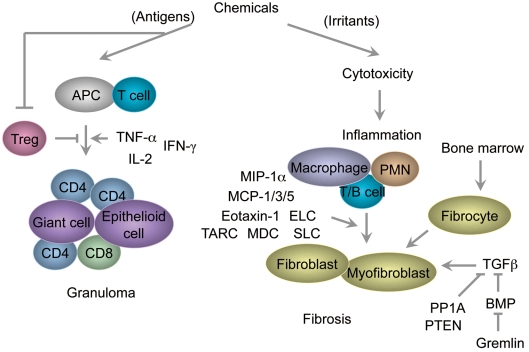
The mechanism by which cytotoxicity and inflammation cause fibrosis in the lung in response to environmental cues is not fully understood. In most pulmonary fibrotic conditions, cytotoxicity, inflammation and repair could be dysregulated by imbalance in the production of chemokines, cytokines and growth factors, and pulmonary wound repair is an extremely dynamic process intersecting immunology, structural biology and airway physiology153. When a cytotoxic effect is minimal or self-limiting, repair would proceed to restore the normal structure, but when an injury is more extensive, tissue repair might result in scarring and/ or fibrosis154. The significance of new discoveries in understanding the pathogenesis of diseases and the potential molecular targets involved in advancing or interfering with fibrogenesis continues to be evaluated using in vivo analyses of animal models substituting for patients with ILDs/ DPLDs155. Important advances have been made in identifying molecules that are able to inhibit or induce lung granulomas and fibrosis, including TGF-β and BMP signaling and chemokines and their receptors in relation to reduction in Tregs (Fig. 2). Evidence by toxicological approaches would be expected to explore new aspects of the pulmonary diseases. Exposure to beryllium causes a Th1 cytokine (TNF-α, IFNγ and IL-2)-dominant granulomatous process, which might share the features of sarcoidosis, a more frequent disorder with similar pathological features156,157. Discovering the role of Tregs in CBD provides new insights in the mechanism of development of granulomatous lesions. On the other hand, nongranulomatous fibrosis has been found in nylon flock-exposed individuals, with or without textile paint exposure, although experimental data are quite limited as of 2010. PVC is known to be an etiological agent of pneumoconiosis, including granulomatous or nongranulomatous fibrosis, and of lung cancer, hepatic angiosarcoma and leukemia/lymphoma158. QACs, including DDAC, may cause pulmonary diseases such as asthma or fibrosis, as shown by etiological research63–65 and our experimental study69. Because there are several types of ILDs/DPLDs (Table 1), there should be a note of caution regarding the pathological characteristics in human and animal cases.
Fig. 2.
Proposed concept of toxicological pulmonary granuloma and fibrosis as lung diseases induced in response to environmental cues. Chemicals have the potential to function as either antigens or irritants or both for alveolar macrophages, dendritic cells and/or epithelial cells. Antigens are presented to T cells by APCs, inducing Th1-type inflammation (expressing TNF-α, IFN-γ and IL-2) and recruiting many CD4+ and CD8+ T cells, which may contribute to the formation of giant and epithelioid cells. Only a few Tregs are found in affected humans and animals as compared with the controls, subsequently disturbing the Th1/Th2 balance. On the other hand, cytotoxicity by irritants induces inflammation in association with the expression of chemokines (MIP-1α, MCP-1, MCP-3, MCP-5, eotaxin-1, TARC, MDC, ELC and SLC) and recruitment of macrophages, PMN and lymphocytes (T cells/B cells), followed by the proliferation of fibroblasts and myofibroblasts. Circulating fibrocytes derived from the bone marrow contribute to fibrotic disorders in the lung. Profibrotic and antifibrotic signaling may be regulated by TGF-β/BMP/gremlin and PP1A/PTEN signaling. APCs, antigen-presenting cells; Th1, T helper 1; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL-2, interleukin-2; Tregs, regulatory T cells; Th2, T helper 2; MIP, macrophage inflammatory protein; MCP-1, monocyte chemotactic protein-1; TARC, thymus- and activation-regulated chemokine; MDC, macrophage-derived chemokine; ELC, Epstein-Barr-induced 1 (EBI1)-ligand chemokine; SLC, secondary lymphoid-tissue chemokine; PMN, polymorphonuclear neutrophils; TGF-β, transforming growth factor-β; BMP, bone morphogenetic protein; PP1A, protein phosphatase 1A; PTEN, phosphatase and tensin homolog.
References
- 1.Greaves P. Respiratory tract. In: Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, Third Edition. Elsevier Inc., Oxford, UK. 215–269. 2007 [Google Scholar]
- 2.King TE., JrClinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 172: 268–279 2005 [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias(This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001). Am J Respir Crit Care Med. 165: 277–304 2002 [DOI] [PubMed] [Google Scholar]
- 4.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 30: 835–839 2007 [DOI] [PubMed] [Google Scholar]
- 5.Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc. 3: 322–329 2006 [DOI] [PubMed] [Google Scholar]
- 6.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 3: 293–298 2006 [DOI] [PubMed] [Google Scholar]
- 7.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 71: 301–326 2004 [DOI] [PubMed] [Google Scholar]
- 8.Kelleher P, Pacheco K, Newman LS. Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect. 108(Suppl 4): 685–696 2000; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper RG, Harrison AP. The uses and adverse effects of beryllium on health. Indian J Occup Environ Med. 13: 65–76 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4+ T cells in patients with chronic beryllium disease. J Clin Invest. 110: 1473–1482 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings KJ, Stefaniak AB, Virji MA, Kreiss K. A reconsideration of acute beryllium disease. Environ Health Perspect. 117: 1250–1256 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bill JR, Mack DG, Falta MT, Maier LA, Sullivan AK, Joslin FG, Martin AK, Freed BM, Kotzin BL, Fontenot AP. Beryllium presentation to CD4+ T cells is dependent on a single amino acid residue of the MHC class II β-chain. J Immunol. 175: 7029–7037 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fontenot AP, Maier LA. Genetic susceptibility and immune-mediated destruction in beryllium-induced disease. Trends Immunol. 26: 543–549 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dai S, Murphy GA, Crawford F, Mack DG, Falta MT, Mar-rack P, Kappler JW, Fontenot AP. Crystal structure of HLA-DP2 and implications for chronic beryllium disease. Proc Natl Acad Sci USA. 107: 7425–7430 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarantino-Hutchison LM, Sorrentino C, Nadas A, Zhu Y, Rubin EM, Tinkle SS, Weston A, Gordon T. Genetic determinants of sensitivity to beryllium in mice. J Immunotoxicol. 6: 130–135 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman LS, Lloyd J, Daniloff E. The natural history of beryllium sensitization and chronic beryllium disease. Environ Health Perspect. 104(Suppl 5): 937–943 1996; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikula KJ, Swafford DS, Hoover MD, Tohulka MD, Finch GL. Chronic granulomatous pneumonia and lymphocytic responses induced by inhaled beryllium metal in A/J and C3H/HeJ mice. Toxicol Pathol. 25: 2–12 1997; . [DOI] [PubMed] [Google Scholar]
- 18.Tinkle SS, Kittle LA, Schumacher BA, Newman LS. Beryllium induces IL-2 and IFN-γ in berylliosis. J Immunol. 158: 518–526 1997 [PubMed] [Google Scholar]
- 19.Sawyer RT, Fontenot AP, Barnes TA, Parsons CE, Tooker BC, Maier LA, Gillespie MM, Gottschall EB, Silveira L, Hagman J, Newman LS. Beryllium-induced TNF-α production is transcription-dependent in chronic beryllium disease. Am J Respir Cell Mol Biol. 36: 191–200 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salehi F, Zayed J, Audusseau S, Muller C, Truchon G, Plamondon P, L’espérance G, Chevalier G, Mazer B. Immunological responses in C3H/HeJ mice following nose-only inhalation exposure to different sizes of beryllium metal particles. J Appl Toxicol. 29: 61–68 2009 [DOI] [PubMed] [Google Scholar]
- 21.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, Fontenot AP. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J Immunol. 180: 2704–2712 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobis DR, Sawyer RT, Gillespie MM, Huang J, Newman LS, Maier LA, Day BJ. Modulation of lymphocyte proliferation by antioxidants in chronic beryllium disease. Am J Respir Crit Care Med. 177: 1002–1011 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comhair SA, Lewis MJ, Bhathena PR, Hammel JP, Erzurum SC. Increased glutathione and glutathione peroxidase in lungs of individuals with chronic beryllium disease. Am J Respir Crit Care Med. 159: 1824–1829 1999; . [DOI] [PubMed] [Google Scholar]
- 24.Kokturk N, Sabag M, Stark M, Grief J, Fireman E. High extracellular induced sputum heme oxygenase-1 in sarcoidosis and chronic beryllium disease. Eur J Clin Invest. 39: 584–590 2009 [DOI] [PubMed] [Google Scholar]
- 25.Inoue Y, King TE, Jr, Tinkle SS, Dockstader K, New-man LS. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am J Pathol. 149: 2037–2054 1996 [PMC free article] [PubMed] [Google Scholar]
- 26.Lehnert NM, Gary RK, Marrone BL, Lehnert BE. Inhibition of normal human lung fibroblast growth by beryllium. Toxicology. 160: 119–127 2001 [DOI] [PubMed] [Google Scholar]
- 27.Coates SS, Lehnert BE, Sharma S, Kindell SM, Gary RK. Beryllium induces premature senescence in human fibroblasts. J Pharmacol Exp Ther. 322: 70–79 2007; . [DOI] [PubMed] [Google Scholar]
- 28.Keshava N, Zhou G, Spruill M, Ensell M, Ong TM. Carcinogenic potential and genomic instability of beryllium sulfate in BALB/c-3T3 cells. Mol Cell Biochem. 222: 69–76 2001 [PubMed] [Google Scholar]
- 29.Jonth AC, Silveira L, Fingerlin TE, Sato H, Luby JC, Welsh KI, Rose CS, Newman LS, du Bois RM, Maier LA. ACCESS GroupTGF-β1 variants in chronic beryllium disease and sarcoidosis. J Immunol. 179: 4255–4262 2007; . [DOI] [PubMed] [Google Scholar]
- 30.Kern DG, Kuhn C, 3rd, Ely EW, Pransky GS, Mello CJ, Fraire AE, Müller J. Flock worker’s lung: broadening the spectrum of clinicopathology, narrowing the spectrum of suspected etiologies. Chest. 117: 251–259 2000 [DOI] [PubMed] [Google Scholar]
- 31.Eschenbacher WL, Kreiss K, Lougheed MD, Pransky GS, Day B, Castellan RM. Nylon flock-associated interstitial lung disease. Am J Respir Crit Care Med. 159: 2003–2008 1999 [DOI] [PubMed] [Google Scholar]
- 32.Boag AH, Colby TV, Fraire AE, Kuhn C, 3rd, Roggli VL, Travis WD, Vallyathan V. The pathology of interstitial lung disease in nylon flock workers. Am J Surg Pathol. 23: 1539–1545 1999 [DOI] [PubMed] [Google Scholar]
- 33.Warheit DB, Hart GA, Hesterberg TW, Collins JJ, Dyer WM, Swaen GM, Castranova V, Soiefer AI, Kennedy GL., JrPotential pulmonary effects of man-made organic fiber (MMOF) dusts. Crit Rev Toxicol. 31: 697–736 2001; . [DOI] [PubMed] [Google Scholar]
- 34.Porter DW, Castranova V, Robinson VA, Hubbs AF, Mercer RR, Scabilloni J, Goldsmith T, Schwegler-Berry D, Battelli L, Washko R, Burkhart J, Piacitelli C, Whitmer M, Jones W. Acute inflammatory reaction in rats after intratracheal instillation of material collected from a nylon flocking plant. J Toxicol Environ Health A. 57: 25–45 1999; . [DOI] [PubMed] [Google Scholar]
- 35.Warheit DB, Webb TR, Reed KL, Hansen JF, Kennedy GL., JrFour-week inhalation toxicity study in rats with nylon respirable fibers: rapid lung clearance. Toxicology. 192: 189–210 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ghio AJ, Funkhouser W, Pugh CB, Winters S, Stonehuerner JG, Mahar AM, Roggli VL. Pulmonary fibrosis and ferruginous bodies associated with exposure to synthetic fibers. Toxicol Pathol. 34: 723–729 2006 [DOI] [PubMed] [Google Scholar]
- 37.Moya C, Antó JM, Taylor AJ. Outbreak of organizing pneumonia in textile printing sprayers. Collaborative Group for the Study of Toxicity in Textile Aerographic Factories. Lancet. 344: 498–502 1994 [DOI] [PubMed] [Google Scholar]
- 38.Romero S, Hernández L, Gil J, Aranda I, Martín C, Sanchez-Payá J. Organizing pneumonia in textile printing workers: a clinical description. Eur Respir J. 11: 265–271 1998 [DOI] [PubMed] [Google Scholar]
- 39.Solé A, Cordero PJ, Morales P, Martínez ME, Vera F, Moya C. Epidemic outbreak of interstitial lung disease in aerographics textile workers–the “Ardystil syndrome”: a first year follow up. Thorax. 51: 94–95 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoet PH, Gilissen LP, Leyva M, Nemery B. In vitro cytotoxicity of textile paint components linked to the “Ardystil syndrome”. Toxicol Sci. 52: 209–216 1999 [DOI] [PubMed] [Google Scholar]
- 41.Clottens FL, Verbeken EK, Demedts M, Nemery B. Pulmonary toxicity of components of textile paint linked to the Ardystil syndrome: intratracheal administration in hamsters. Occup Environ Med. 54: 376–387 1997; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoet PH, Gilissen L, Nemery B. Polyanions protect against the in vitro pulmonary toxicity of polycationic paint components associated with the Ardystil syndrome. Toxicol Appl Pharmacol. 175: 184–190 2001 [DOI] [PubMed] [Google Scholar]
- 43.Soutar CA, Copland LH, Thornley PE, Hurley JF, Ottery J, Adams WG, Bennett B. Epidemiological study of respiratory disease in workers exposed to polyvinylchloride dust. Thorax. 35: 644–652 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lilis R. Review of pulmonary effects of poly(vinyl chloride) and vinyl chloride exposure. Environ Health Perspect. 41: 167–169 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordasco EM, Demeter SL, Kerkay J, Van Ordstrand HS, Lucas EV, Chen T, Golish JA. Pulmonary manifestations of vinyl and polyvinyl chloride (interstitial lung disease). Newer aspects. Chest. 78: 828–834 1980 [DOI] [PubMed] [Google Scholar]
- 46.Arnaud A, Pommier de Santi PP, Garbe L, Payan H, Charpin J. Polyvinyl chloride pneumoconiosis. Thorax. 33: 19–25 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antti-Poika M, Nordman H, Nickels J, Keskinen H, Viljanen A. Lung disease after exposure to polyvinyl chloride dust. Thorax. 41: 566–567 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White NW, Ehrlich RI. Regression of polyvinylchloride polymer pneumoconiosis. Thorax. 52: 748–749 1997; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groth DH, Lynch DW, Moorman WJ, Stettler LE, Lewis TR, Wagner WD, Kommineni C. Pneumoconiosis in animals exposed to poly(vinyl chloride) dust. Environ Health Perspect. 41: 73–81 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H, Dinsdale D, Nemery B, Hoet PH. Role of residual additives in the cytotoxicity and cytokine release caused by polyvinyl chloride particles in pulmonary cell cultures. Toxicol Sci. 72: 92–102 2003 [DOI] [PubMed] [Google Scholar]
- 51.Xu H, Verbeken E, Vanhooren HM, Nemery B, Hoet PH. Pulmonary toxicity of polyvinyl chloride particles after a single intratracheal instillation in rats. Time course and comparison with silica. Toxicol Appl Pharmacol. 194: 111–121 2004 [DOI] [PubMed] [Google Scholar]
- 52.Xu H, Vanhooren HM, Verbeken E, Yu L, Lin Y, Nemery B, Hoet PH. Pulmonary toxicity of polyvinyl chloride particles after repeated intratracheal instillations in rats. Elevated CD4/CD8 lymphocyte ratio in bronchoalveolar lavage. Toxicol Appl Pharmacol. 194: 122–131 2004; . [DOI] [PubMed] [Google Scholar]
- 53.Gannon PF, Burge PS, Benfield GF. Occupational asthma due to polyethylene shrink wrapping (paper wrapper’s asthma). Thorax. 47: 759 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuomainen A, Stark H, Seuri M, Hirvonen MR, Linnainmaa M, Sieppi A, Tukiainen H. Experimental PVC material challenge in subjects with occupational PVC exposure. Environ Health Perspect. 114: 1409–1413 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skaliy P, Thompson TA, Gorman GW, Morris GK, McEachern HV, Mackel DC. Laboratory studies of disinfectants against Legionella pneumophila. Appl Environ Microbiol. 40: 697–700 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanazawa A, Ikeda T, Endo T. Synthesis and antimicrobial activity of dimethyl- and trimethyl-substituted phosphonium salts with alkyl chains of various lengths. Antimicrob Agents Chemother. 38: 945–952 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickey P. Guidelines for selecting wood preservatives. For the San Francisco Department of the Environment. September 9, 2003. [The guidelines are available at http://www. sfenvironment.org/downloads/library/preservatives.pdf].
- 58.Walsh SE, Maillard JY, Russell AD, Catrenich CE, Charbonneau DL, Bartolo RG. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J Appl Microbiol. 94: 240–247 2003 [DOI] [PubMed] [Google Scholar]
- 59.United States Environmental Protection Agency Reregistration eligibility decision for aliphatic alkyl quaternaries (DDAC). August, 2006. [The guidelines are available at http://www.epa.gov/oppsrrd1/REDs/ddac_red.pdf].
- 60.Yoshimatsu T, Hiyama K. Mechanism of the action of didecyldimethylammonium chloride (DDAC) against Escherichia coil and morphological changes of the cells. Biocontrol Sci. 12: 93–99 2007 [DOI] [PubMed] [Google Scholar]
- 61.Ioannou CJ, Hanlon GW, Denyer SP. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob Agents Chemother. 51: 296–306 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dejobert Y, Martin P, Piette F, Thomas P, Bergoend H. Contact dermatitis from didecyldimethylammonium chloride and bis-(aminopropyl)-lauryl amine in a detergent-disinfectant used in hospital. Contact Dermatitis. 37: 95–96 1997 [DOI] [PubMed] [Google Scholar]
- 63.Bernstein JA, Stauder T, Bernstein DI, Bernstein IL. A combined respiratory and cutaneous hypersensitivity syndrome induced by work exposure to quaternary amines. J Allergy Clin Immunol. 94: 257–259 1994 [DOI] [PubMed] [Google Scholar]
- 64.Burge PS, Richardson MN. Occupational asthma due to indirect exposure to lauryl dimethyl benzyl ammonium chloride used in a floor cleaner. Thorax. 49: 842–843 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purohit A, Kopferschmitt-Kubler MC, Moreau C, Popin E, Blaumeiser M, Pauli G. Quaternary ammonium compounds and occupational asthma. Int Arch Occup Environ Health. 73: 423–427 2000 [DOI] [PubMed] [Google Scholar]
- 66.Vincent G, Kopferschmitt-Kubler MC, Mirabel P, Pauli G, Millet M. Sampling and analysis of quaternary ammonium compounds (QACs) traces in indoor atmosphere. Environ Monit Assess. 133: 25–30 2007 [DOI] [PubMed] [Google Scholar]
- 67.Shirai J, Kanno T, Inoue T, Mitsubayashi S, Seki R. Effects of quaternary ammonium compounds with 0.1% sodium hydroxide on swine vesicular disease virus. J Vet Med Sci. 59: 323–328 1997 [DOI] [PubMed] [Google Scholar]
- 68.Shirai J, Kanno T, Tsuchiya Y, Mitsubayashi S, Seki R. Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses. J Vet Med Sci. 62: 85–92 2000 [DOI] [PubMed] [Google Scholar]
- 69.Ohnuma A, Yoshida T, Tajima H, Fukuyama T, Hayashi K, Yamaguchi S, Ohtsuka R, Sasaki J, Fukumori J, Tomita M, Kojima S, Takahashi N, Takeuchi Y, Kuwahara M, Takeda M, Kosaka T, Nakashima N, Harada T. Didecyldimethylammonium chloride induces pulmonary inflammation and fibrosis in mice. Exp Toxicol Pathol. 62: 643–651 2010 [DOI] [PubMed] [Google Scholar]
- 70.Toxicity Profile BIBRA. Didecyldimethylammonium Chloride. BIBRA International Ltd., Carshalton, UK, 1990 [Google Scholar]
- 71.Xue Y, Hieda Y, Saito Y, Nomura T, Fujihara J, Takayama K, Kimura K, Takeshita H. Distribution and disposition of benzalkonium chloride following various routes of administration in rats. Toxicol Lett. 148: 113–123 2004; . [DOI] [PubMed] [Google Scholar]
- 72.Swiercz R, Hałatek T, Wasowicz W, Kur B, Grzelińska Z, Majcherek W. Pulmonary irritation after inhalation exposure to benzalkonium chloride in rats. Int J Occup Med Environ Health. 21: 157–163 2008 [DOI] [PubMed] [Google Scholar]
- 73.Tokuda A, Itakura M, Onai N, Kimura H, Kuriyama T, Matsushima K. Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J Immunol. 164: 2745–2751 2001 [DOI] [PubMed] [Google Scholar]
- 74.Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitter-ding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 167: 4368–4377 2001 [DOI] [PubMed] [Google Scholar]
- 75.Yara S, Kawakami K, Kudeken N, Tohyama M, Teruya K, Chinen T, Awaya A, Saito A. FTS reduces bleomycininduced cytokine and chemokine production and inhibits pulmonary fibrosis in mice. Clin Exp Immunol. 124: 77–85 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huaux F, Gharaee-Kermani M, Liu T, Morel V, McGarry B, Ullenbruch M, Kunkel SL, Wang J, Xing Z, Phan SH. Role of Eotaxin-1 (CCL11) and CC chemokine receptor 3 (CCR3) in bleomycin-induced lung injury and fibrosis. Am J Pathol. 167: 1485–1496 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, Matsushima K, Mukaida N. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 170: 843–854 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol. 83: 237–244 2008 [DOI] [PubMed] [Google Scholar]
- 79.Hasegawa M, Sato S, Takehara K. Augmented production of chemokines (monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1a (MIP-1α) and MIP-1β) in patients with systemic sclerosis: MCP-1 and MIP-1α may be involved in the development of pulmonary fibrosis. Clin Exp Immunol. 117: 159–165 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C–C chemokines in fibrotic lung disease. J Leukoc Biol. 57: 782–787 1995 [DOI] [PubMed] [Google Scholar]
- 81.Emad A, Emad V. Elevated levels of MCP-1, MIP-α and MIP-1β in the bronchoalveolar lavage (BAL) fluid of patients with mustard gas-induced pulmonary fibrosis. Toxicology. 240: 60–69 2007 [DOI] [PubMed] [Google Scholar]
- 82.Shinoda H, Tasaka S, Fujishima S, Yamasawa W, Miyamoto K, Nakano Y, Kamata H, Hasegawa N, Ishizaka A. Elevated CC chemokine level in bronchoalveolar lavage fluid is predictive of a poor outcome of idiopathic pulmonary fibrosis. Respiration. 78: 285–292 2009 [DOI] [PubMed] [Google Scholar]
- 83.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 29: 313–326 2009; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 271: 17779–17784 1996 [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1α loop. J Immunol. 164: 6174–6179 2000 [DOI] [PubMed] [Google Scholar]
- 86.Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 3: 357–363 2006 [DOI] [PubMed] [Google Scholar]
- 87.Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine. 24: 266–276 2003 [DOI] [PubMed] [Google Scholar]
- 88.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C–C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 204: 594–604 2004; . [DOI] [PubMed] [Google Scholar]
- 89.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, 3rd, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/ CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 284: L342–L349 2003 [DOI] [PubMed] [Google Scholar]
- 90.Reichel CA, Khandoga A, Anders HJ, Schlöndorff D, Luck-ow B, Krombach F. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tis sue. J Leukoc Biol. 79: 114–122 2006 [DOI] [PubMed] [Google Scholar]
- 91.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 166: 675–684 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 185: 99–109 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 35: 175–181 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 117: 902–909 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 87: 1047–1082 2007 [DOI] [PubMed] [Google Scholar]
- 96.Shang X, Qiu B, Frait KA, Hu JS, Sonstein J, Curtis JL, Lu B, Gerard C, Chensue SW. Chemokine receptor 1 knockout abrogates natural killer cell recruitment and impairs type-1 cytokines in lymphoid tissue during pulmonary granuloma formation. Am J Pathol. 157: 2055–2063 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blease K, Mehrad B, Standiford TJ, Lukacs NW, Kunkel SL, Chensue SW, Lu B, Gerard CJ, Hogaboam CM. Airway remodeling is absent in CCR1–/– mice during chronic fungal allergic airway disease. J Immunol. 165: 1564–1572 2000 [DOI] [PubMed] [Google Scholar]
- 98.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 12: 313–335 2001 [DOI] [PubMed] [Google Scholar]
- 99.Belperio JA, Dy M, Murray L, Burdick MD, Xue YY, Strieter RM, Keane MP. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J Immunol. 173: 4692–4698 2004 [DOI] [PubMed] [Google Scholar]
- 100.Trujillo G, O’Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol. 172: 1209–1221 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Misson P, van den Brûle S, Barbarin V, Lison D, Huaux F. Markers of macrophage differentiation in experimental silicosis. J Leukoc Biol. 76: 926–932 2004 [DOI] [PubMed] [Google Scholar]
- 102.Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, Lundblad LK, Norton R, van der Vliet A, Janssen-Heininger YM. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 181: 4255–4264 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 164: 1315–1326 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 174: 1745–1755 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi ES, Pierce EM, Jakubzick C, Carpenter KJ, Kunkel SL, Evanoff H, Martinez FJ, Flaherty KR, Moore BB, Toews GB, Colby TV, Kazerooni EA, Gross BH, Travis WD, Hogaboam CM. Focal interstitial CC chemokine receptor 7 (CCR7) expression in idiopathic interstitial pneumonia. J Clin Pathol. 59: 28–39 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Evanoff H, Flaherty KR, Martinez FJ, Toews GB, Hogaboam CM. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur Respir J. 29: 1082–1093 2007 [DOI] [PubMed] [Google Scholar]
- 107.Trujillo G, Hartigan AJ, Hogaboam CM. T regulatory cells and attenuated bleomycin-induced fibrosis in lungs of CCR7–/– mice. Fibrogenesis Tissue Repair. 3: 18 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sobiecka M, Kus J, Demkow U, Filewska M, Jozwik A, Radwan-Rohrenschef P, Chorostowska-Wynimko J. Induced sputum in patients with interstitial lung disease: a non-invasive surrogate for certain parameters in bronchoalveolar lavage fluid. J Physiol Pharmacol. 59(Suppl 6): 645–657 2008 [PubMed] [Google Scholar]
- 109.Feghali-Bostwick CA, Tsai CG, Valentine VG, Kantrow S, Stoner MW, Pilewski JM, Gadgil A, George MP, Gibson KF, Choi AM, Kaminski N, Zhang Y, Duncan SR. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 179: 2592–2599 2007 [DOI] [PubMed] [Google Scholar]
- 110.van Oosterhout AJ, Bloksma N. Regulatory T-lymphocytes in asthma. Eur Respir J. 26: 918–932 2005 [DOI] [PubMed] [Google Scholar]
- 111.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 276: 37672–37679 2001 [DOI] [PubMed] [Google Scholar]
- 112.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 4: 330–336 2003 [DOI] [PubMed] [Google Scholar]
- 113.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299: 1057–1061 2003 [DOI] [PubMed] [Google Scholar]
- 114.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 4: 337–342 2003 [DOI] [PubMed] [Google Scholar]
- 115.Mack DG, Lanham AM, Palmer BE, Maier LA, Fontenot AP. CD27 expression on CD4+ T cells differentiates effector from regulatory T cell subsets in the lung. J Immunol. 182: 7317–7324 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, Bouros D. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 179: 1121–1130 2009 [DOI] [PubMed] [Google Scholar]
- 117.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, Duncan SR. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 5: e8959 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taflin C, Miyara M, Nochy D, Valeyre D, Naccache JM, Altare F, Salek-Peyron P, Badoual C, Bruneval P, Haroche J, Mathian A, Amoura Z, Hill G, Gorochov G. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol. 174: 497–508 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verrecchia F, Mauviel A. Transforming growth factorß and fibrosis. World J Gastroenterol. 13: 3056–3062 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koli K, Myllärniemi M, Vuorinen K, Salmenkivi K, Ryynänen MJ, Kinnula VL, Keski-Oja J. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 169: 61–71 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors-β1, -β2, and -β3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 150: 981–991 1997 [PMC free article] [PubMed] [Google Scholar]
- 122.Eickelberg O, Köhler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-β1 and TGF-β3. Am J Physiol. 276: L814–L824 1999 [DOI] [PubMed] [Google Scholar]
- 123.Bobik A. Transforming growth factor-βs and vascular disorders. Arterioscler Thromb Vasc Biol. 26: 1712–1720 2006 [DOI] [PubMed] [Google Scholar]
- 124.Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGF-β superfamily signaling. FASEB J. 13: 2105–2124 1999 [PubMed] [Google Scholar]
- 125.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 18: 816–827 2004; . [DOI] [PubMed] [Google Scholar]
- 126.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 282: L585–L593 2002 [DOI] [PubMed] [Google Scholar]
- 127.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-β-mediated pulmonary fibrosis. J Immunol. 173: 2099–2108 2004 [DOI] [PubMed] [Google Scholar]
- 128.Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor β1 stimulation of α2(I)-collagen (COL1A2) transcription. J Biol Chem. 275: 39237–39245 2000 [DOI] [PubMed] [Google Scholar]
- 129.Xu Y, Sengupta PK, Seto E, Smith BD. Regulatory factor for X-box family proteins differentially interact with his-tone deacetylases to repress collagen α2(I) gene (COL1A2) expression. J Biol Chem. 281: 9260–9270 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu Y, Farmer SR, Smith BD. Peroxisome proliferatoractivated receptor γ interacts with CIITA·RFX5 complex to repress type I collagen gene expression. J Biol Chem. 282: 26046–26056 2007 [DOI] [PubMed] [Google Scholar]
- 131.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, Shi Y, Chen YG, Meng A, Feng XH. PPM1A functions as a Smad phosphatase to terminate TGF-β signaling. Cell. 125: 915–928 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bu S, Kapanadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-β/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem. 283: 19593–19602 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, Schäfer-Korting M, Pfeilschifter J, Huwiler A. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-binduced cell responses. J Biol Chem. 279: 35255–35262 2004 [DOI] [PubMed] [Google Scholar]
- 134.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-b1. Am J Respir Cell Mol Biol. 37: 395–404 2007 [DOI] [PubMed] [Google Scholar]
- 135.Zhao J, Shi W, Chen H, Warburton D. Smad7 and Smad6 differentially modulate transforming growth factor β-induced inhibition of embryonic lung morphogenesis. J Biol Chem. 275: 23992–23997 2000 [DOI] [PubMed] [Google Scholar]
- 136.Inoue Y, Imamura T. Regulation of TGF-β family signaling by E3 ubiquitin ligases. Cancer Sci. 99: 2107–2112 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor β signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 27: 4488–4499 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Venkatesan N, Pini L, Ludwig MS. Changes in Smad expression and subcellular localization in bleomycininduced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 287: L1342–L1347 2004 [DOI] [PubMed] [Google Scholar]
- 139.Gonzalez AV, Le Bellego F, Ludwig MS. Imbalance of receptor-regulated and inhibitory Smads in lung fibroblasts from bleomycin-exposed rats. Am J Respir Cell Mol Biol. 36: 206–212 2007 [DOI] [PubMed] [Google Scholar]
- 140.Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 104: 5–11 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wen FQ, Liu X, Kobayashi T, Abe S, Fang Q, Kohyama T, Ertl R, Terasaki Y, Manouilova L, Rennard SI. Interferon-γ inhibits transforming growth factor-β production in human airway epithelial cells by targeting Smads. Am J Respir Cell Mol Biol. 30: 816–822 2004 [DOI] [PubMed] [Google Scholar]
- 142.Shukla MN, Rose JL, Ray R, Lathrop KL, Ray A, Ray P. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol. 40: 643–653 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 168: 629–638 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res. 97: 496–504 2005 [DOI] [PubMed] [Google Scholar]
- 145.Izumi N, Mizuguchi S, Inagaki Y, Saika S, Kawada N, Nakajima Y, Inoue K, Suehiro S, Friedman SL, Ikeda K. BMP-7 opposes TGF-β1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol. 290: L120–L126 2006 [DOI] [PubMed] [Google Scholar]
- 146.Kowanetz M, Valcourt U, Bergström R, Heldin CH, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol Cell Biol. 24: 4241–4254 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 16: 1987–2002 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Myllärniemi M, Lindholm P, Ryynänen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 177: 321–329 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murray LA, Hackett TL, Warner SM, Shaheen F, Argentieri RL, Dudas P, Farrell FX, Knight DA. BMP-7 does not protect against bleomycin-induced lung or skin fibrosis. PLoS One. 3: e4039 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pegorier S, Campbell GA, Kay AB, Lloyd CM. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-b1 in normal human lung fibroblasts (NHLF). Respir Res. 11: 85 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koli K, Wempe F, Sterner-Kock A, Kantola A, Komor M, Hofmann WK, von Melchner H, Keski-Oja J. Disruption of LTBP-4 function reduces TGF-β activation and enhances BMP-4 signaling in the lung. J Cell Biol. 167: 123–133 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Costello CM, Cahill E, Martin F, Gaine S, McLoughlin P. Role of gremlin in the lung: development and disease. Am J Respir Cell Mol Biol. 42: 517–523 2010 [DOI] [PubMed] [Google Scholar]
- 153.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2: 103–121 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res. 3: 1 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 33: 9–13 2005 [DOI] [PubMed] [Google Scholar]
- 156.Richeldi L. Chronic beryllium disease: a model for pulmonary sarcoidosis? Acta Biomed. 76(Suppl 2): 11–14 2005 [PubMed] [Google Scholar]
- 157.Chen ES, Moller DR. Expression profiling in granulomatous lung disease. Proc Am Thorac Soc. 4: 101–107 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gennaro V, Ceppi M, Crosignani P, Montanaro F. Reanalysis of updated mortality among vinyl and polyvinyl chloride workers: confirmation of historical evidence and new findings. BMC Public Health. 8: 21 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]