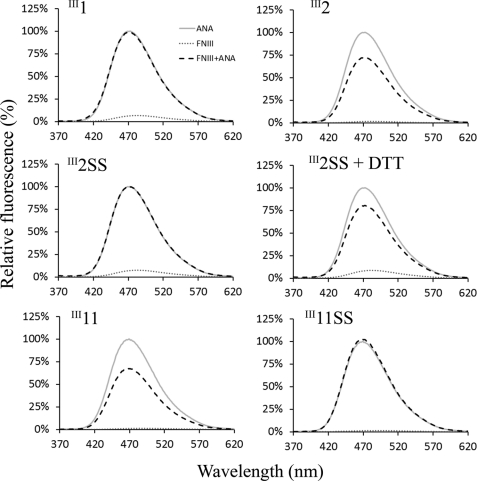
FIGURE 4.
FNIII domain binding to anastellin measured by ANS fluorescence. 10 μm anastellin, 10 μm FNIII domains, and mixtures of anastellin and FNIII domains were mixed with 50 μm ANS. ANS fluorescence was recorded from 370 to 620 nm, with excitation at 360 nm. The emission intensity was normalized to that of the mixture of ANS and anastellin at 470 nm. ANS reacted with anastellin to enhance its fluorescence, but this was decreased by the presence of III2 and III11, indicating that these domains interacted with anastellin to reduce ANS binding. The disulfide bonds in III2 and III11 eliminated anastellin binding. Under reducing conditions, the disulfide mutants interacted with anastellin and led to a reduction in ANS fluorescence just like wild type.