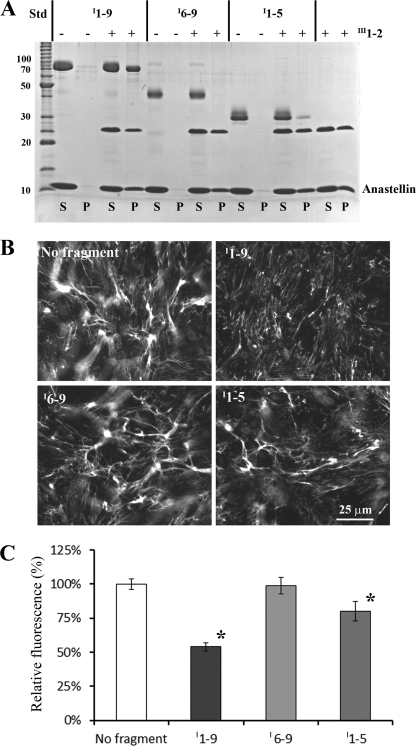
FIGURE 6.
Activities of I1–9, I6–9, and I1–5 for binding the III1–2/anastellin aggregate and inhibiting FN matrix formation. A, for the pelleting assay, 2 μm I1–9, I6–9 (∼40 kDa), or I1–5 (∼30 kDa) was mixed with 40 μm anastellin, with or without 10 μm III1–2. A significant amount of I1–9 co-precipitated with the III1–2/anastellin aggregate. On the other hand, only a trace amount of I1–5 co-precipitated, and I6–9 did not co-precipitate at all. None of the fragments formed any aggregates with anastellin in the absence of III1–2. S, supernatant; P, pellet; Std, BenchMark protein ladder (Invitrogen). B, various FN fragments (1 μm) were added to FN(−/−) cell culture with FN-YPet (30 nm) and cultured for ∼16 h. I1–9 inhibited FN matrix formation, I6–9 had no significant effect on FN matrix assembly, and I1–5 reduced the FN matrix. C, the amount of matrix FN was estimated by measuring YPet fluorescence after solubilizing YPet from cell culture by trypsin treatment. The emission intensity was normalized to that of a control sample (no fragment). I1–9 and I1–5 reduced the FN matrix by 50 and 20%, respectively. The error bars indicate standard deviation. *, p < 0.01.