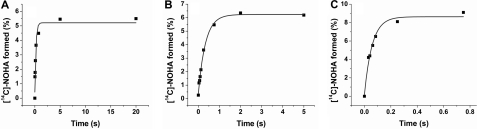
FIGURE 8.
Kinetics of NOHA formation from l-Arg by rapid-quench experiments and HPLC analysis. Product formation was monitored by the formation of [14C]NOHA from l-[14C]Arg as described under “Experimental Procedures.” Mutant W66H catalyzed the conversion of l-Arg at a slower rate than wild-type BsNOS. In contrast, W66F displayed a faster conversion of the substrate to form NOHA compared with the native protein. These results are in agreement with the trends observed during single turnover reactions with l-Arg and H4T, and suggest that the mutations had a substantial impact on the kinetic properties of these proteins.