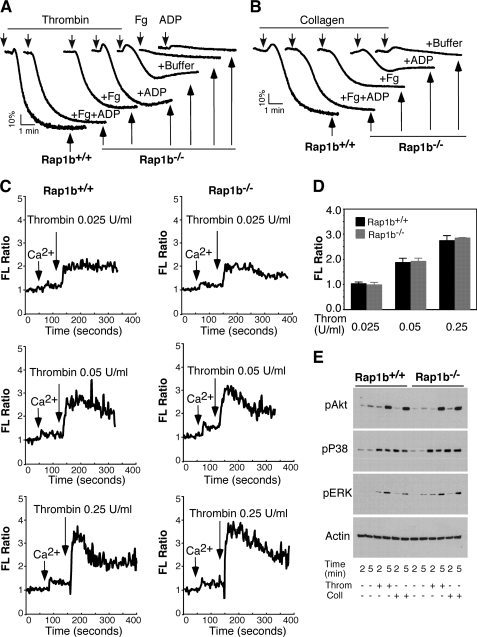
FIGURE 4.
ADP and fibrinogen rescued aggregation of Rap1b-deficient platelets, and the role of Rap1b in calcium mobilization. A and B, washed platelets from Rap1b+/+ (Rap1b+/+) and Rap1b−/− mice (Rap1b−/−) were added with 0.025 unit/ml thrombin (A) or 1.0 μg/ml collagen (B) in the absence (buffer) or presence of ADP (0.5 μm) (+ADP), fibrinogen (50 μg/ml) (+Fg), or both (+Fg+ADP) to induce aggregation. Platelets were also added with fibrinogen (Fg) or ADP (ADP) alone (A). Aggregation traces shown are representative of at least three independent experiments. C and D, washed platelets from Rap1b-deficient or wild-type mice were labeled with 12.5 μm Fura-2/AM/0.2% Pluronic F-127 and resuspended in Tyrode's solution. Platelets were then stimulated with various concentration of thrombin. Changes in the intracellular free calcium level were measured every 2 s and expressed as a ratio of fluorescence (FL) detected at 509 nm emission with an excitation wavelength of 340 nm and 380 nm (C). Statistical data from three experiments are shown (D). E, washed platelets from Rap1b+/+ and Rap1b−/− mice were added with 0.025 unit/ml thrombin (Throm) or 1.0 μg/ml collagen (Coll), and incubated at 37 °C in the aggregometer for 2 or 5 min. Platelets were solubilized in 1 × SDS sample buffer, and phosphorylation of Akt, p38, and ERK was detected by Western blot using phospho-specific rabbit monoclonal antibodies against phosphorylated Ser473 of Akt, Thr180/Tyr182 of p38, and Thr202/Tyr204 of ERK (Cell Signaling Technologies). A mouse monoclonal antibody against β-actin (Sigma) was used to verify equal loading.