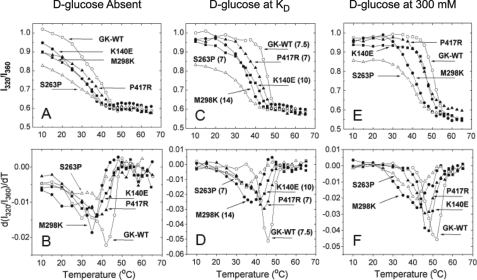
FIGURE 3.
Temperature denaturation of control and mutant GCKs measured by tryptophan fluorescence. The results of five different enzymes are as follows: 1) white circles, stable control; 2) moderately unstable mutants: black squares GCKK140E and black triangles GCKP417R; and 3) severely unstable mutants: white triangles GCKS263P and black circles GCKM298K, which have relative activity indices of 0.65 and 0.58, respectively, extrapolating to thresholds of GSIR of about 6 mm. The ratios of 320 nm to 360 nm portions of the fluorescence spectra are shown in the upper panels, and the differential forms in the lower panels. A. and B, in d-glucose-free buffer; C and D with d-glucose at the respective KD in mm; E and F, at 300 mm d-glucose. The excitation wavelength was 295 nm. Samples contained 5 mm phosphate (pH 7.3), 100 mm KCl, 1 mm DTT, and 3 mm enzyme.