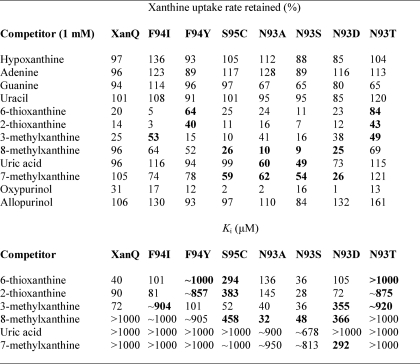
TABLE 2.
Specificity profile of mutants at positions Asn-93, Phe-94, and Ser-95
E. coli T184 expressing the corresponding constructs were assayed for initial rates of [3H]xanthine (1 μm) uptake at 5–20 sec, in the absence or presence of the indicated unlabeled nucleobases and analogues. For initial assessment of the inhibitory effect, xanthine (1 μm) uptake assays were performed in the presence of a 1000-fold excess (1 mm) of unlabeled competitor and the percentage of the uptake rate retained was determined. The uptake value obtained in the absence of competitor was taken as 100%. Values represent the means of three determinations, with standard deviations <20%. For kinetic inhibition analysis, xanthine (1 μm) transport assays were performed in the presence of 0.001–2 mm of competitor and IC50 and Ki values were determined as described under “Experimental Procedures.” Most significant differences from the wild-type profile are highlighted in bold. All mutants and the wild-type XanQ version used contained a C-terminal BAD. The mutants used are in the wild-type background except S95C, which is in the C-less permease background. The low affinity observed with S95C for 8-methylxanthine (Ki 458 μm) is attributable to a comparably low affinity observed with C-less which distinguishes C-less from wild-type XanQ (not shown; see also Ref. 6).