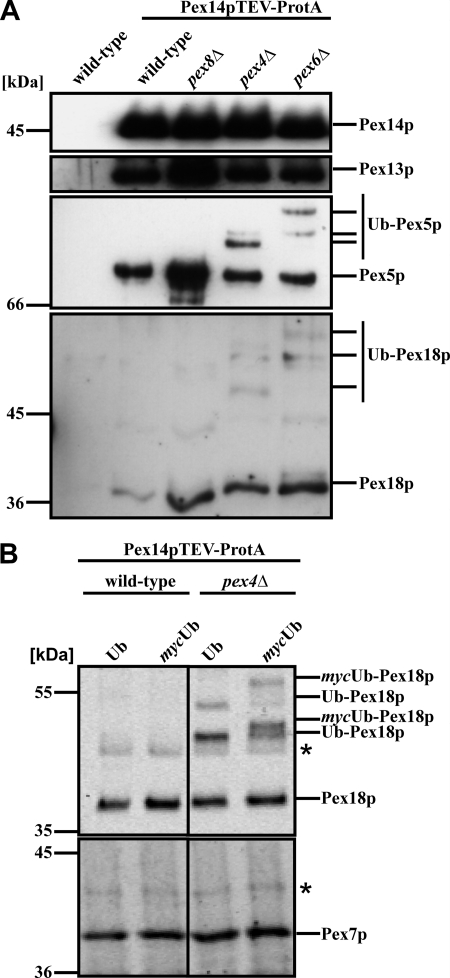
FIGURE 2.
Ubiquitination of Pex18p. Pex14p-TEV-ProtA-complexes were isolated by affinity chromatography from membrane fractions of the indicated strains. A, eluate fractions were analyzed by immunoblotting for the presence of Pex14p, Pex13p, and Pex18p as well as the PTS1 receptor Pex5p. Higher molecular weight species of Pex18p were detected in strains deficient in either PEX4 or PEX6, which resembled the appearance of ubiquitinated Pex5p (Ub-Pex5p) in these strains. B, Pex14p-TEV-ProtA complexes were isolated from wild-type and pex4Δ cells expressing either plasmid-encoded wild-type Ub or mycUb. In pex4Δ-cells, Pex18p modifications with a higher molecular weight were visible, which shifted to protein species of even lower electrophoretic mobility upon expression of mycUb, demonstrating that these modifications represent ubiquitinated Pex18p.