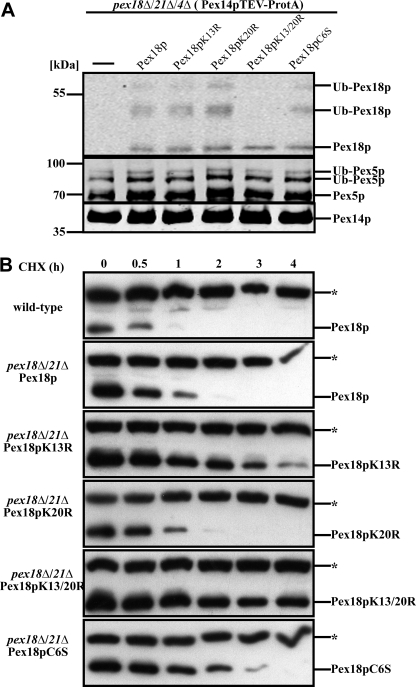
FIGURE 4.
Lysines 13 and 20 of Pex18p are required for co-receptor polyubiquitination and proteasomal degradation. A, Pex14p complexes were isolated from pex18Δ/21Δ/4Δ and pex18Δ/21Δ/4Δ cells expressing the indicated Pex18p variants. Eluate fractions were inspected for the presence of Pex14p, Pex18p, and Pex5p by immunoblot analysis. In all samples, Pex5p displayed the ubiquitination pattern typical for pex4Δ cells. Although different Pex18p variants exhibited a normal modification pattern, no ubiquitinated Pex18p species were detected when both lysine 13 and 20 were changed to arginine (Pex18pK13R/K20R). This finding identifies both lysine residues as targets for ubiquitin attachment. B, wild-type as well as pex18Δ21Δ cells expressing the indicated Pex18p variants were grown on oleic acid for 14 h and treated with 15 μg/ml cycloheximide. At the indicated time points, samples were taken and analyzed for Pex18p by immunoblotting. *, cross-reactive band. Although wild-type Pex18p was below the detection level after 1 h, Pex18pK13R/K20R was almost stable along the whole time period. These data indicate that the conserved lysine residues are required for proteasomal degradation of Pex18p and support the notion that they provide the target residues for polyubiquitination.