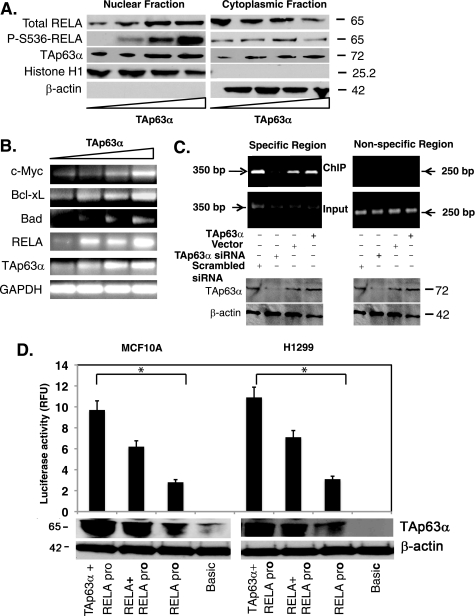
FIGURE 5.
TAp63α mediated the RelA transcriptional regulation. MCF10A cells were transfected with the TAp63α expression plasmid (0–1.5 μg). A, immunoblotting of the nuclear and cytosolic fractions with indicated antibodies. B, semi-qRT-PCR analysis for mRNA levels of indicated genes. C, ChIP assay performed with MCF10A cells, 72 h after transfection with the TAp63α expression construct or an empty vector, as well as with the TAp63α siRNA or scrambled siRNA. Total lysates aliquots of the same samples (for specific and nonspecific regions) were tested for both TAp63α and β-actin levels using immunoblotting, as indicated. TAp63α-bound RelA promoter DNA was precipitated with the 4A4 antibody followed by the PCR for the specific (−1900 to −1551bp; supplemental Fig. S1) and nonspecific regions (−600 to −351bp; supplemental Fig. S1), designated as ChIP. PCR with the purified DNA (Input) for both regions is shown. D, MCF10A (left) and H1299 (right) cells were transfected with the basic TA-luc and RelA-luc constructs along with the Renilla luciferase plasmid, with or without the TAp63α and/or RelA expression cassette for 24 h. The firefly luciferase activity was normalized for the Renilla luciferase activity. Immunoblotting for TAp63α and β-actin was performed. Data are presented as relative -fold change units (RFU) from the basic TA-luc activity designated as 1. *, p ≤ 0.001(n = 5) compared with basic TA-luc activity or RelA-luc activity alone.