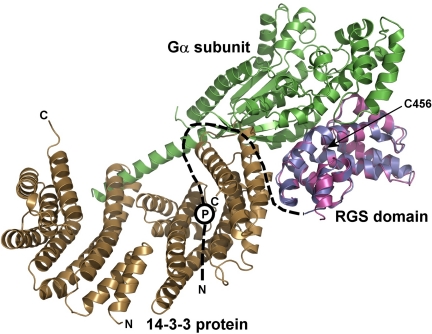
FIGURE 7.
The 14-3-3 protein binding sterically occludes the Gα binding interface of the RGS domain of RGS3. The RGS domain of the 14-3-3ζ·pRGS3 complex (shown in blue) is superimposed with the RGS domain of the Giα1·RGS4 complex crystal structure (shown in magenta) (6). The missing N-terminal part of RGS3 is schematically shown as a black dashed line. The approximate position of the phosphorylation site Ser-264 is depicted as a circled P. The Gα subunit is shown in green. As can be seen, the 14-3-3 protein would sterically occlude the Gα·RGS3 complex formation due to its binding in close proximity to the Gα binding interface of the RGS domain (indicated by steric clashes between the 14-3-3ζ protein and Gα). The position of Cys-456 is indicated with an arrow.