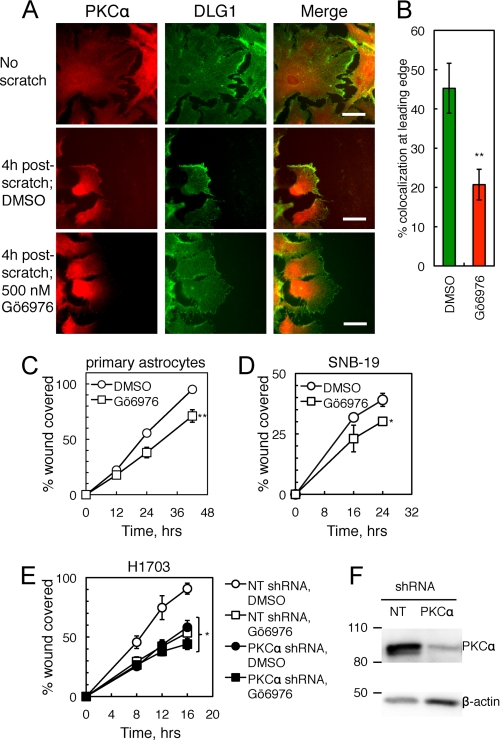
FIGURE 2.
PKCα and DLG1 promote cell migration in wound-healing assays. A, PKCα and DLG1 colocalization at the leading edge of migrating astrocytes is shown. Primary murine astrocytes were pretreated with Gö6976 (500 nm; bottom row) or a corresponding amount of DMSO (middle row), scratched with a 10-μl pipette tip, fixed 4 h after scratch, and stained for endogenous PKCα and DLG1. Scale bars: 50 nm. B, shown is quantification of the percent of cells with colocalization of PKCα and DLG1 at the leading edge of migrating cells (n = 5 experiments with >30 cells per condition in each experiment). C–E, shown is the effect of cPKC inhibition on wound healing. Primary astrocytes (C), SNB-19 glioblastoma cells (D), and stable non-targeting shRNA-expressing (NT; open symbols) or PKCα shRNA-expressing (filled symbols) cell lines derived from H1703 NSCLC cells (E) were pretreated with mitomycin C (to inhibit proliferation) followed by DMSO (circles) or Gö6976 (squares), scratched with a pipette tip, and followed over 12–42,16–24, or 8–16 h, respectively. The area covered by migrating cells was quantified using ImageJ; data points represent the mean ± S.E. of at least three experiments. F, a Western blot shows PKCα knockdown efficiency for a representative experiment as in E. After completion of the scratch assay, cells were lysed, and lysates were probed for PKCα and the loading control β-actin. *, significantly different from control DMSO-treated cells, p < 0.05; **, p < 0.01.